
Courses

By Shailendra Singh
|
Updated on 3 Jun 2025, 14:52 IST
The nitrogen cycle is an integral part of the ecosystem. In this article, we will consider its effects on the environment in more detail. In addition, nitrogen is an important nutrient for plants. However, much of the nitrogen in the atmosphere cannot be used directly by plants or animals. Continue reading to explore how the Nitrogen cycle makes usable nitrogen available to plants and other organisms.
The nitrogen cycle can be described as one of the biogeochemical cycles that converts unused nitrogen into the atmosphere into a viable form of nitrogen. Before we go on to discuss the nitrogen cycle, we need to know some facts about nitrogen. Nitrogen is an essential element of all living things. Nitrogen atoms can be found in all proteins and nucleic acids. Nitrogen is usually present as nitrogen gas (N2) in the environment. Nitrogen fixation is the process by which bacteria convert gaseous nitrogen into ammonia, a type of nitrogen that can be used by plants.
Plants provide nitrogen molecules to animals when they are eaten. In both nature and agriculture, nitrogen is a relatively common nutrient. Limited nutrition is one that has a limited amount and prevents growth. It is therefore clear that nitrogen is an essential element of nature. The topic focuses on the details of the nitrogen cycle.
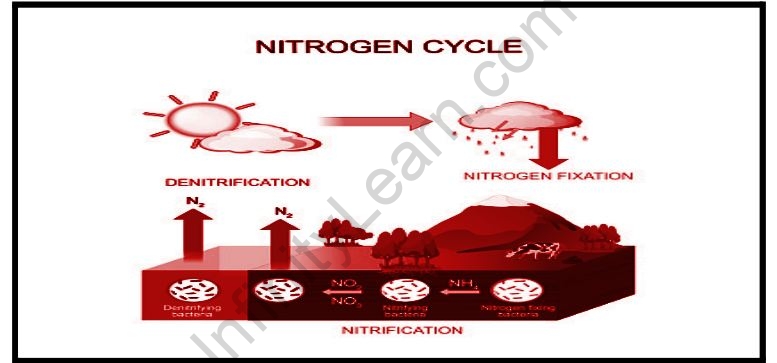
The Nitrogen Cycle is a biogeochemical process in which nitrogen is converted into many forms, passing from the atmosphere to the soil into an organism and returning to the atmosphere. It involves several processes such as nitrogen fixation, nitrification, denitrification, decomposition and decomposition.
Nitrogen gas is present in both organic and inorganic species. Organic nitrogen is present in living things, and it passes through the food chain through the use of other organisms. Inorganic nitrogen species are abundant in the atmosphere. This nitrogen is made available to plants by symbiotic bacteria that can convert inactive nitrogen into usable species – such as nitrites and nitrates.
Nitrogen undergoes various modifications to maintain balance in the ecosystem. Moreover, this process extends to various biomes, the ocean nitrogen cycle is one of the most complex biogeochemical cycles.
It is the first step in the nitrogen cycle. Here, Atmospheric nitrogen (N2), which is mainly derived from an inactive form, is converted into an active form of ammonia (NH3).

During nitrogen fixation, an inert type of nitrogen gas is absorbed into the soil from the atmosphere and surface water, especially by precipitation. Later, nitrogen undergoes a series of chemical reactions, in which two nitrogen atoms are separated and mixed with hydrogen to form ammonia (NH4 +).
The entire Nitrogen repair process is eliminated by symbiotic bacteria called Diazotrophs. Azotobacter and Rhizobium also play a key role in this process. These bacteria combine to form a powerful nitrogenase enzyme that combines nitrogen gas and hydrogen to form ammonia.
Nitrogen fixation is possible by atmospheric adjustment- which includes light or industrial adjustment by producing ammonia under high temperatures and pressure conditions. This can also be remedied by man-made processes, especially industrial processes that create fertilizers rich in ammonia and nitrogen.
In this process, ammonia is converted into nitrate by the presence of bacteria in the soil. Nitrites are formed by the oxidation of ammonia with the help of species Nitrosomonas bacteria. Later, the nitrites produced were converted into nitrates by Nitrobacter. This conversion is very important as ammonia gas is toxic to plants.
The reaction involved in the Nitrification process is as follows:
2NH4 + + 3O2 → 2NO2– + 4H + + 2H2O

2NO2– + O2 → 2NO3–
Main producers – plants take nitrogen compounds from the soil with the help of their roots, which are found in the form of ammonia, nitrite ions, nitrate ions, or ammonium ions and are used in the formation of the plant and animal proteins. In this way, it gets into the web of food where the main consumers are eating vegetables.
When plants or animals die, the nitrogen in the organism returns to the soil. Decaying organisms, which are bacteria or fungi present in the soil, convert organic matter into ammonium. This process of decomposition produces ammonia, which is used in other biological processes.
Denitrification is the process by which nitrogen compounds return to the atmosphere by converting nitrate (NO3-) into nitrogen (N). This process of the nitrogen cycle is a final process and occurs when there is no oxygen. Denitrification is made up of a type of divisive bacteria – Clostridium and Pseudomonas, which will process nitrate for oxygen and provide free nitrogen gas as a by-product.
The nitrogen cycle process occurs in the same way in the marine ecosystem as in the terrestrial ecosystem. The only difference is that it is caused by bacteria in the ocean.
Nitrogen-containing compounds fall into the sea as the residues are long pressurized and form sedimentary rock. Due to the geological height, these sedimentary rocks move to the ground. Initially, it was not known that these sedimentary rocks containing nitrogen were an important source of nitrogen. However, recent studies have confirmed that nitrogen from these rocks is released into plants as a result of rocky weather.
The importance of the nitrogen cycle is as follows:
Nitrogen forms many cellular elements and is essential for many biological processes. For example, amino acids contain nitrogen and makeup building blocks that make up various parts of the human body such as hair, tissues, and muscles.
Plants need nitrogen as this element is an important component of chlorophyll. Therefore, chlorophyll is essential for the photosynthesis process, so nitrogen deficiency can cause deficiency, strong growth, and other abnormalities.
Ammonification occurs during biological decomposition when ammonifying bacteria convert organic nitrogen into inanimate components such as ammonia or ammonium ions.