
Courses

By Shailendra Singh
|
Updated on 23 Jan 2025, 15:08 IST
Isotopes are two or maybe more groups of atoms that share the same atomic number and periodic table position but have distinct nucleon numbers due to differing neutron counts. Even though the chemical properties of all isotopes of the same element are almost identical, their atomic weights and physical attributes differ. An element’s isotopes have the same number of protons but differ in the number of neutrons.
The former denotes that isotopically distinct compounds experience the same reactions, but the latter denotes that their mass differs. An isotope is a chemical element that has a different number of neutrons in its nucleus than its parent element. Most elements have several isotopes. Many are stable, but others are radioactive or only exist for a short time before decomposing into other elements.
Isotope ratios have been important in many fields of science, and they’ve been utilized to figure out the Earth’s age and history, as well as the Solar System’s origins. From nuclear power to medicine and carbon dating, radioactive isotopes have a wide range of applications.
The information about isotopes from various physics-related articles is available here. Isotope and its general concepts are important topics in physics. Students who want to flourish in physics need to be well known about isotopes to get deep knowledge about them to do well on their exams. The definition, brief explanation, types, and applications are provided here to assist students in effectively understanding the respective topic.
In general, isotopes are said to be atoms with variable numbers of neutrons but the same number of protons and electrons in the same element. The total number of neutrons in different isotopes of an element varies means that the masses of the isotopes differ. The superscript number to the left of the element designation indicates the number of protons plus neutrons in the isotope.
The atomic number is the total number of protons in an atom’s nucleus and the number of electrons in a neutral (non-ionized) atom’s nucleus. Each atomic number identifies a specific element, but not an isotope; the number of neutrons in a given element’s atom can vary significantly. The amount of nucleons (both protons and neutrons) in an atom’s nucleus determines its mass number, thus each isotope of a given element has a distinct mass number.
Frederick Soddy, a radiochemist, was the one who discovered the isotopes. Despite the modern periodic chart only allowing for 11 elements between lead and uranium included, investigations of radioactive decay chains showed around 40 distinct species referred to as radioelements (i.e. radioactive elements) between uranium and lead in 1913.

The nucleus shows radioactivity as a result of nuclear instability. It is the process of heavy elements disintegrating into comparably lighter ones by the emission of radiation. In 1896, Henri Becquerel discovered it. Radiation released from an atom’s unstable nucleus causes energy to be lost. The force of repulsion, also known as electrostatic force, and the nucleus’s forces of attraction, which maintain the nucleus together, are the driving forces behind this occurrence. In the natural world, these two forces are thought to be immensely powerful.
The nucleus shows radioactivity as a result of nuclear instability. It is the process of heavy elements disintegrating into comparably lighter ones by the emission of radiation. In 1896, Henri Becquerel discovered it. Radiation released from an atom’s unstable nucleus causes energy to be lost. The force of repulsion, also known as electrostatic force, and the nucleus’s forces of attraction, which maintain the nucleus together, are the driving forces behind this occurrence. In the natural world, these two forces are thought to be immensely powerful.
The attribute of naturally occurring elements and artificially created isotopes of the elements is radioactive decay. The half-life of a radioactive element, which is the time it takes for one-half of any given quantity of the isotope to decay, is used to describe how quickly it decays.
Isotopes of hydrogen and carbon are common examples. If we consider hydrogen, there are three stable isotopes: protium, deuterium, and tritium. Apparently, protium, deuterium, and tritium have almost the same number of protons but different numbers of neutrons: protium has zero, deuterium has one, and tritium has two.
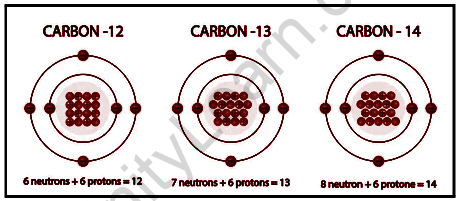
Whenever it comes to carbon, there are three isotopes: Carbon-12, Carbon-13, and Carbon-14. Here, the atomic masses of the given isotopes are 12, 13, and 14. Carbon-12 is a stable isotope in this situation, whereas carbon-14 is primarily a radioactive isotope.
Other common isotopes include: Tin has 22 isotopes, Zinc has 21 known isotopes, Neon is a mixture of 3 isotopes, natural xenon is a mixture of 9 stable isotopes, and Nickel has 14 known isotopes.

Isotope refers to one of two or more species of atoms in a chemical element that share the same atomic number and periodic table position, as well as virtually identical chemical behavior, but differ in atomic weights and physical attributes. Each and every chemical element has one or more isotopes
DNA damage can be caused by inhaling radioisotopes. In the stomach, radioactive isotopes can irradiate for a long time. Extremely high doses can cause sterility or mutations in the body. Skin cancer and burns can both be caused by radiation.
Generally, isotopes are said to be atoms with variable numbers of neutrons but the same number of protons and electrons in the same element. However, radioactive isotopes have nuclei that spontaneously decay overtime to generate other isotopes, making them unstable.
The average time it takes for half of an atom’s isotope to decay is called its half-life.