
Courses

By Shailendra Singh
|
Updated on 4 Dec 2024, 11:44 IST
Nature of Carbonyl Group:A carbonyl group is a chemically organic functional group composed of a carbon atom double-bonded to an oxygen atom –> [C=O] The most superficial carbonyl groups are aldehydes and ketones usually attached to another carbon compound.
These structures can be planted in multiplex sweet mixtures contributing to smell and taste. There are two main types of carbonyl compounds: organic and inorganic. Here, we will discuss organic carbonyl compounds.
The carbonyl group is the organic functional group composed of the carbon atom that has a double bond with the oxygen atom. Ketones and aldehydes are the most superficial carbonyl groups, and usually, they are attached to another carbon compound. In any perfumed mixture, their structure can be planted, and they have a significant donation to the taste and the smell of the scented composites.
The situation where carbon makes the double bond with the oxygen is considered the carbonyl group entity, and the group members are known as the carbonyl compounds. Generally, the carbon and oxygen of the carbonyl group are planar and sp2 hybridized.
Also Read: Time Management Strategy for the NEET Exam
The carbon-oxygen bond is polarized due to the high electronegativity difference between carbon and oxygen titles of the C =O bond.
Oxygen has an advanced electronegativity compared to that of the carbon particle. Wherefore, the carbonyl carbon demonstrates the property of a Lewis acid, whereas the carbonyl oxygen demonstrates the property of a Lewis base.
Carbonyl compounds contain significant dipole moments. Therefore, it demonstrates more polarity than ethers. The high opposition of the carbonyl group is substantially due to the resonance concerning neutral and dipolar structures.
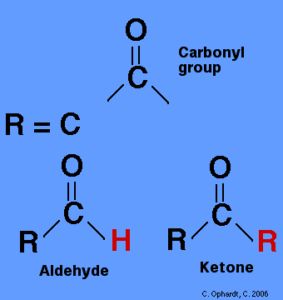
Carbonyl Compounds definition can be given as the compounds that hold a carbonyl group (the C=O group) are known as carbonyl compounds.

The carbonyl grouping is presumably the essential functional group of organic chemistry. These are an integral part of organic chemistry, and their primary members are called ketones, carboxylic acids, and aldehydes.
Carbonyl mixtures are further divided into organic and inorganic carbonyl combinations. This complete article details the organic carbonyl compounds.
Carbonyl groups are the functional groups of aldehydes and ketones. Carbonyl groups are located at the end of aldehydes, while carbonyl groups are located in the middle of ketones.
Molecular rotation is possible because of the double bond of carbonyl groups. Nature contains various types of carbonyl compounds. The acyl groups (C-R = O) all have a substituent attached. R consists of an alkyl, an alkenyl, an alkynyl, or a combination of these functional groups.
Examples of Carbonyl Compounds
Carbamates, urea, acyl chlorides, chloroformates, carbonate esters, lactones, thioesters, lactams, isocyanates, and hydroxamates.
Metal carbonyls are cooperation complexes of transition matter with carbon monoxide ligands. The general formula of material carbonyls is Mx (CO) y. A lone couple of electrons are available on both carbon and oxygen tittles of a carbon monoxide ligand. As the carbon titles present electrons to the essence, these complexes are carbonyls.
Mononuclear essence carbonyls similar as Fe (CO) 2, Ni (CO)4, and binuclear essence carbonyls similar to Co2 (CO) 8 may be prepared by the direct response of CO with finely divided essence at suitable temperature and pressure.
The main requirement of this method is that the metal centre must be in a reduced low oxidation state to facilitate CO binding to the metal centre through metal to ligand π–back donation.
Treating salts like Ru(acac)3, CrCl3, VCl3, CoS, Col2 with carbon monoxide in the presence of suitable reducing agents like Mg, Ag, Cu, Na, H2, LiAlH4, etc. gives metal carbonyls.
When a cold answer of Fe (CO) 5/ naughts (CO) 5 in numbing acetic acid CH3COOH is irradiated with ultraviolet light, Fe2 (CO) 9/ Os2 (CO) 9 are carried.
Carbonyl compounds are organic compounds containing a carbonyl group (C=O), where a carbon atom is double-bonded to an oxygen atom. The nature of carbonyl compounds is characterized by their ability to act as both electrophilic and nucleophilic sites. They are highly reactive due to the polar nature of the carbonyl group, where the carbon atom carries a partial positive charge and the oxygen atom carries a partial negative charge.
The carbonyl group is weakly acidic. The oxygen atom in the carbonyl group has lone pairs of electrons that can participate in protonation, especially under acidic conditions. In a reaction, the carbonyl group can be protonated (making it more electrophilic) or form bonds with nucleophiles. However, the carbonyl group itself is generally more acidic than other parts of the molecule due to its ability to donate electrons to stabilize a positive charge (in certain reactions).
The carbonyl group (C=O) consists of a carbon atom double-bonded to an oxygen atom. The carbonyl carbon is electrophilic, making it an ideal site for nucleophilic attack. The carbonyl group has a linear structure, with bond angles of about 120°. The oxygen atom is more electronegative than the carbon atom, causing the bond to be polar, with the oxygen carrying a partial negative charge and the carbon carrying a partial positive charge.
The ketone group consists of a carbonyl group (C=O) attached to two carbon atoms. Ketones are slightly less reactive than aldehydes because the alkyl groups attached to the carbonyl carbon donate electron density through inductive effects, which somewhat stabilizes the electrophilic character of the carbonyl carbon. However, ketones are still electrophilic and can undergo nucleophilic addition reactions.
CO (carbon monoxide) is generally considered to be basic. It can act as a nucleophile in reactions, due to its lone pair of electrons on the carbon atom, which can form bonds with electrophilic species. CO can also act as a ligand to metal centers in metal carbonyl complexes.
The carboxyl group (-COOH) is acidic. The hydrogen atom attached to the oxygen of the carboxyl group can be readily dissociated, forming a carboxylate ion (RCOO⁻). The resulting negative charge on the oxygen is stabilized by resonance, making the carboxyl group an effective acid. Therefore, carboxylic acids tend to lose a proton (H⁺) in aqueous solutions.