
Courses

By Shailendra Singh
|
Updated on 20 Nov 2024, 15:12 IST
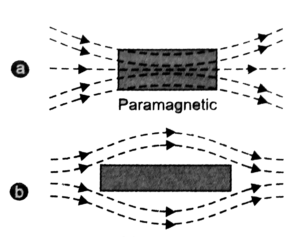
Paramagnetic Material is a property of certain materials that causes them to be weakly attracted to magnetic fields. When these materials are exposed to an external magnetic field, internal induced magnetic fields form that are ordered in the same direction as the applied field.
When the applied field is removed, the materials lose their magnetism because thermal motion randomizes the electron spin orientations. Paramagnetically active materials are referred to as paramagnetic.
Under certain conditions, some compounds and most chemical elements are paramagnetic. On the other hand, true paramagnets exhibit magnetic susceptibility according to the Curie or Curie-Weiss laws and paramagnetism over a wide temperature range. Myoglobin, transition metal complexes, iron oxide (FeO), and oxygen are all examples of paramagnets (O2).
Titanium and aluminium are paramagnetic metallic elements. Superparamagnets are materials with a net paramagnetic response but microscopic ferromagnetic or ferrimagnetic ordering.
These materials obey the Curie law while having extremely large Curie constants. Superparamagnets are examples of ferrofluids. Mictomagnets are another name for solid superparamagnets. A micromagnetic is an alloy such as AuFe (gold-iron). The alloy’s ferromagnetically coupled clusters freeze below a certain temperature.
Because unpaired electrons exist in paramagnetic materials, the net magnetic moment of all electrons in an atom does not equal zero. As a result, an atomic dipole exists in this case. When an external magnetic field is applied, the atomic dipole aligns in the direction of the external magnetic field. Paramagnetic materials are thus weakly magnetised in the direction of the magnetising field. In layman’s terms, these materials typically have a weak attraction to magnets. Paramagnetism is the name given to this type of magnetism. It is caused primarily by unpaired electrons in the material or by the partial alignment of randomly oriented atomic dipoles along the field.
M = χH = C/T x H

Where,
M stands for magnetization.
χ= susceptibility to magnetic fields,
C denotes the material-specific Curie constant.
T denotes the absolute (Kelvin) temperature.
H denotes the auxiliary magnetic field.

Metals that are weakly attracted to magnets are known as paramagnetic materials. Aluminium, gold, and copper are among them. These substances' atoms contain electrons, the majority of which spin in the same direction... but not all. This imparts polarity to the atoms.
Unpaired electrons have a magnetic dipole moment due to their spin and act like tiny magnets. When an external magnetic field is applied, the spins of the electrons align parallel to the field, resulting in a net attraction. Aluminium, oxygen, titanium, and iron oxide are examples of paramagnetic materials (FeO).
Paramagnetic substances are solids that are weakly attracted by a magnetic field. They are magnetically oriented in the same direction as the magnetic field. These aren't strong magnets. When a magnetic field attracts one or more unpaired electrons, paramagnetism occurs.