
Courses

By Shailendra Singh
|
Updated on 27 Nov 2024, 16:39 IST
Animes are organic compositions that include and are positioned on one or more nitrogen atoms. Amines are built to resemble ammonia, with the nitrogen capable of bonding with three hydrogens. Amphetamines, on the other hand, have unique properties due to their carbon connectivity. Organic substituents such as alkyl and aryl, for example, can replace one or more hydrogen atoms from ammonia in an anime. Anemone is a type of organic molecule that contains nitrogen but is not an anime. It has carboxylic acid derivatives in the ground state that contain trivalent ammonia. Amines and amides are very different in terms of their characteristics and compositions, so understanding their distinction is critical. Amines are classified into three types: primary, secondary, and tertiary. The number of carbon-containing groups connected to an amine determines its type. An amine is classified as primary if it contains one carbon-containing molecule, secondary if it contains two carbon-containing molecules, and tertiary if it contains three carbon-containing molecules.
Also Check: Absorptive & Emissive Power
Diazonium salts were first synthesised in 1858 from aromatic amines, and the dye industry quickly recognised their utility in the production of azo compounds. Colours from the visible spectrum are added to dyes suitable for a variety of fibres by modifying the chemical structures of the diazotized amines (the diazo components) and the compounds with which they react (the coupling components). The diazonium group can be replaced by a variety of atoms or groups of atoms, often with the assistance of copper or a copper salt; these reactions allow for the preparation of a wide range of aromatic derivatives. Hydrazine derivatives are formed through the chemical reduction of aromatic diazonium salts. Diazonium compounds, also known as diazonium salts, are a class of organic compounds that share the functional group R−N+2X−, where R can be any organic group, such as an alkyl or an aryl, and X can be an inorganic or organic anion, such as a halogen.
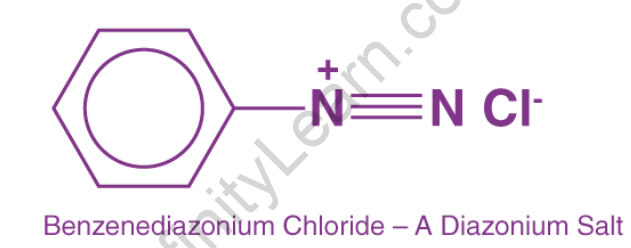
Diazonium salts are important organic salts distinguished by the presence of the -N2+X- group, where X can be any organic or inorganic anion. They are called ‘Di-Azonium’ because their cationic part contains two nitrogen atoms (N2). They are represented chemically as R-N2+X-, where R can be any alkyl or aryl group.
The diazonium group is easily replaced by a variety of functional groups, including –I, –OH, –F, –CN, and –H, which cannot be substituted directly into the aromatic ring. Furthermore, replacement patterns that are diametrically opposed to the norm can be generated (i.e., preparation of 1,3-dihalo substituted benzenes). The diazonium salt is not separated in the majority of these replacements. Arenediazonium salts are a type of chemical that contains arene diazonium. The word di refers to two, aza to nitrogen, and onium to the ionic nature of the compound in the phrase “Diazonium salts.” As a result, diazonium salts are ionic compounds that contain N≡N. Diazonium salts are organic compounds that have triple bonds between Nitrogen atoms and either an alkyl or an aryl (benzene ring) on the other side. Diazonium salts are the transitional phase between azo dyes (or compounds are known to be popular colouring agents). Salts are named after the double nitrogen (diazo) found in ionic salts, where chloride molecules replace the nitrogen atom.
Also Check: Improvement in Food Production
As a result, they have two nitrogen atoms, one of which is charged. Diazonium salts include benzenediazonium chloride (C6H5N2+Cl–), benzene diazonium hydrogen sulphate (C6H5N2+HSO4–), and others. Diazonium salts are one of the most versatile organic and inorganic component combinations. It is generally represented as RN2+X. The R represents an organic group, usually an aryl group, and the X represents an ion. X in diazonium salts is typically Cl–, Br–, or BF4.
The presence of the N2+ group or the diazonium group gives rise to the names of these salts. The suffix diazonium is appended to the parent hydrocarbon from which these salts are derived, followed by the anion X, such as bromide.

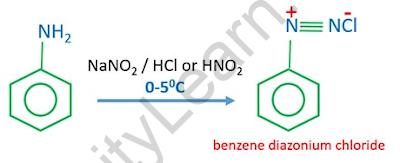
At 273–278 degrees Celsius, aniline reacts with nitrous acid to form benzene diazonium chloride. Nitrous acid is formed in the reaction mixture when sodium nitrite reacts with hydrochloric acid. The diazotisation process involves the conversion of primary aromatic amines into diazonium ions. Because of its volatility, the diazonium salt is rarely stored and is used immediately after production.
NaNO2+HCl→HNO2+NaCl
HNO2+HNO2→N+=O+H2O+NO2–
Diazonium salts are created through a process known as diazotization. It is also known as dissociation at times. Diazonium Salts are typically prepared using primarily Aromatic Amines. Other types of organic compounds, however, may also be used. The most common method for converting Aromatic Amines into Diazonium Salts is a diazotization reaction between the Aromatic Amine (acting as a base) and Nitrous Acid (acting as an acid).
A diazonium salt is an organic compound with a nitrogen-nitrogen triple bond and another generic side group that could be alkyl (an alkane derivative) or aryl (benzene ring). The salt part of the name refers to the fact that the diazo (meaning ‘di-nitrogen’) portion of the compound exists as an ionic salt, with a chloride ion serving as a common counter-ion for positively charged nitrogen atoms. The production of diazonium salts is a straightforward procedure. Using an alkyl or a primary arylamine and reacting it with sodium nitrite in the presence of hydrochloric acid will do the trick nicely. Most of the time, the reaction conditions are mild and can be run at room temperature or even lower if necessary.

Also Check: The Moment of Inertia
Aniline is used to make benzene diazonium chloride. When aniline reacts with nitrous acid at low temperatures (0-50 C), the product is benzene diazonium chloride. When the temperature rises, benzene diazonium chloride decomposes into phenol. As a result, when preparing benzene diazonium chloride, you must exercise caution with regard to temperature.
Reactions caused by the substitution of one group for the diazonium group
Benzene Diazonium chloride (C6H5-N2Cl) can be converted into a variety of important aromatic organic compounds, including chlorobenzene, bromobenzene, phenol, and others. The -N2Cl group is replaced with another group such as -Cl, -Br, -OH, and so on.
Benzene diazonium chloride Sandmeyer reactions
Traugott Sandmeyer, a Swiss chemist, discovered SandMeyer reactions in 1884. As a result of Sandmeyer reactions, we can produce chloribenzene, bromebenzene, benzonitrile, iodobenzene, and flurobenzene.
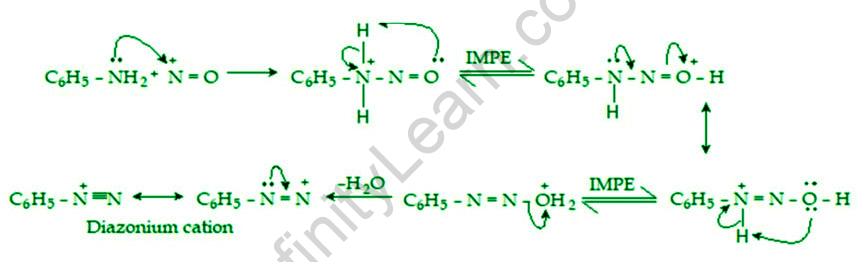
The nitrosonium cation, formed by the reaction of nitrous acid with acid, is the reactive species in diazotization. The nitrogen of the nucleophilic amine is then nitrosated by the electrophilic nitrosonium cation. Diazonium salt is formed as a result of a series of protonic shifts followed by water loss. The mechanism of diazotization begins with the reaction of nitrous acid with the other acid, which produces water and nitrosonium ions. As a result, the necessary nitrosonium ion is formed. The aromatic ring to which the NH2 group is attached is now reacted with this. As a new nitrogen-nitrogen bond is formed, the positive charge of the nitrosonium ion is transferred to the nitrogen, which is now directly attached to the aromatic ring. The n-nitrosamine is produced as a result of the subsequent deprotonation. In the presence of excess acid, n-nitrosamine can be protonated and then deprotonated to form diazohydroxide. To produce the required aryl diazonium ion, diazohydroxide is protonated, and water is removed from the compound (which can easily be converted into a diazonium salt).
Diazotization, also known as dissociation, is the process of converting an organic compound, most commonly primary aromatic amines, into diazonium salts. Diazonium groups are extremely unstable and thus cannot be stored. As a result, we usually use them right away after preparing them. The reaction of nitrous acid with aromatic amines is one of the most common methods of producing diazonium salt.
Diazonium Salts have a unique ability to degrade in the presence of ultraviolet light. This property is used during the reproduction process of a document.
Diazonium salts are most stable at temperatures ranging from 0 to 5 degrees Celsius. In higher temperatures, the Diazonium ion usually decomposes to give off Nitrogen.