
Courses

By Shailendra Singh
|
Updated on 21 Nov 2024, 15:04 IST
It is observed that metals and their alloys are widely used in everyday life. They are used in the manufacture of machines, railways, automobiles, bridges, buildings, agricultural tools, aircraft, and ships, among other things. As a result, large-scale production of a variety of metals is required for a country’s economic growth. Only a few metals, such as gold, silver, and mercury, occur naturally in their free state. The majority of the other metals, on the other hand, are found in the earth’s crust in combined form, i.e. as compounds with various anions such as oxides, sulfides, halides, and so on. In light of this, the study of metal recovery from ores is critical. A series of processes are used to extract metals from their ores. The stages differ depending on the type of ore, the reactivity of the metal, and the nature of the impurities in the ore. The processes involved in metal extraction and refinement are referred to as metallurgy. Most metal ores must be transported to the Earth’s surface in order for metal to be extracted. This procedure is known as mining. In general, the extraction of metals is divided into three stages.
Electrometallurgy tends to dominate aluminum extraction, is important in magnesium production, and has numerous applications in copper and another metal refining. Electron transfer provides a pathway for reduction and oxidation reactions to occur at different locations in many metallothermic reduction processes, increasing reaction area and improving kinetics. Because of its superconformal filling ability, electroplating is used for integrated circuit copper metallization on a much smaller scale rather than evaporation or chemical vapor deposition. The use of electrons to change the oxidation state of ions and atoms is common to all of these processes.
It is known that the most reactive metals are at the top of the metal reactivity series, while the least reactive metals are at the bottom. Metals with the lowest reactivity, such as gold, platinum, and silver, exist in their natural state in the Earth’s crust. However, in their ore, these metals can be found with sulfide minerals such as pyrites, chalcopyrites, and so on. Other low reactive metals, such as mercury, can be found in cinnabar ore as its sulfide.
Metals in the middle of the series, such as zinc and iron, are moderately reactive and exist in the Earth’s crust as oxides, sulfides, and carbonates. Zinc, for example, occurs as a sulfide in zinc blende ore and as carbonate in smithsonite ore, whereas iron occurs as sulfides in iron pyrite ore and as an oxide in magnetite ore.
The most reactive metals, such as sodium, can be found in rock salt ore as chloride and in Chile saltpeter as nitrate. As a result, metals can be divided into three categories based on their reactivity: metals with low reactivity, metals with moderate reactivity, and metals with high reactivity.
There are several steps involved in the extraction of metals from their respective ores, including ore concentration, roasting, calcination, reduction, electrolysis, and refining. Metals with varying reactivities are extracted in a variety of ways.
Metals are most abundant in the earth’s crust. Metal soluble salts can also be found in seawater. Metals exist in nature in a variety of forms, some of which are free, but the majority of which are combined. A metal’s natural mode of occurrence is largely determined by its nature. Those metals which are least reactive and have little or no affinity for oxygen, moisture, and other chemical reagents exist in their free, metallic, or native state, i.e., uncombined state. Because most metals are reactive, they are found in combined form, i.e. as compounds. Minerals are the natural substances found in the earth that contain metals or their compounds. The mineral has a distinct chemical composition. It could be a single compound or a complex mixture of compounds. Ores are the minerals from which metals can be extracted in a convenient and cost-effective manner. All ores are minerals, but not all minerals are ores. Bauxite and clay, for example, are both aluminum minerals. It is bauxite, not clay, that is used in the extraction of aluminum. As a result, bauxite is an ore of aluminum. Ores are classified into different types.

The following are some of the most common steps in the extraction of metals from their ores:
(i) Crushing and pulverization: The ore is typically obtained in the form of large rock fragments. These large ore lumps are crushed into smaller pieces using jaw crushers and grinders. Crushed ore is easier to work with. The ore is brought in between the plates of a crusher, forming a jaw. One of the crusher’s plates is stationary, while the other moves back and forth, and the crushed pieces are collected below.
The crushed ore is then pulverized (powdered) in a stamp mill. To powder the ore, the heavy stamp rises and falls on a hard die. A stream of water is then used to extract the powdered ore through a screen. A ball mill can also be used for pulverization. The crushed ore is placed in a steel cylinder filled with iron balls. The cylinder has been set in motion. The impact of the striking balls pulverizes the crushed ore into fine powder.
(ii) Concentration or dressing of the ore: In general, the ores are found mixed with earthy impurities such as sand, clay, limestone, and so on. Such undesirable impurities in the ore are referred to as gangue or matrix.
Concentration, also known as ore dressing, is the process of removing gangue from powdered ore. Concentrating the ores can be done in a variety of ways. The method used is determined by the nature of the ore.
(iii) Calcination or roasting of the ore: Calcination or roasting is used to convert the concentrated ore into metal oxide.

Calcination is said to be the process of heating concentrated ore in a limited supply of air to remove moisture, the water of hydration, and gaseous volatile substances. The ore is heated to a temperature that prevents it from melting.
Roasting is a method that entails heating the concentrated ore in a free supply of air to a temperature that is insufficient to meet it.
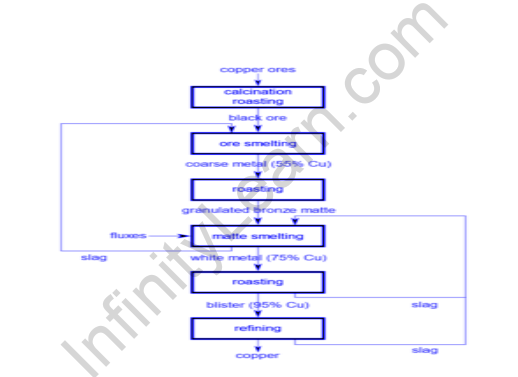
(iv) Reduction of metal oxides to free metal: This procedure is carried out after the ores have been calcined or roasted. The oxide ores are reduced into the metallic state in this process known as smelting.
A few metals cannot be extracted from their ores using common reducing agents like C, CO, H2, and so on. In such cases, other methods of reduction are employed.
(v) Purification and refining of metal: Metals produced by any other method, with the exception of electrolytic reduction, are generally impure. Impurities can take the form of other metals, unreduced metal oxide, nonmetals such as carbon, silicon, phosphorus, sulfur, and so on, and flux or slag. Methods include liquation, poling, distillation, and electrolytic refining.
Metal ions can be reduced to their respective metals in solution (or) molten states using metallurgical electrochemical principles. The reduction is accomplished through electrolysis (or) the use of reducing elements. These techniques are based on electrochemical principles.[Metal-ion + reducing element gives Metal + Reduced ion]
Electrochemical techniques are indeed a class of methods used to investigate the composition and properties of a medium using electrochemical reactions that occur at the electrode-solution interface. The basic principle of electrochemical techniques is to use an appropriate device, usually an electrode or an electrode pair, to convert one chemical parameter of a medium into an electrical parameter, and then to measure this electrical parameter using an instrument. Electrochemical techniques, like electrical measurements, can make use of four parameters. The electrode potential may be affected by the solution’s composition. Potentiometry refers to techniques for measuring this potential. An electrical current may flow through the circuit when an external voltage is applied to an electrode. Voltammetry refers to techniques that make use of the current-voltage relationship. Conductometry is a technique for measuring the electrical resistance (conductance) between two electrodes in a medium. The electrode is the site of electrochemical reactions in all of these techniques, and it is the most important element in electrochemical analyses. As a result, in the discussion, the electrode potential at the electrode-solution interface is first examined when no current flows, and then the effect of current on electrode potential, electrode polarization, is discussed.
The electricity-powered decomposition reaction extracts metals from naturally occurring compounds such as oxides and chlorides.
Rocks and soils are the primary natural sources of heavy metals in the environment. The main rocks, also known as magmatic or igneous rocks, crystallise as magma cools.
Metals can be extracted from ore in three ways. The methods used are electrolysis, reducing an ore with a more reactive metal, and reducing the ore with carbon.