
Courses

By Shailendra Singh
|
Updated on 16 May 2025, 12:52 IST
Boric acid has the chemical formula H3BO3 and is a monobasic Lewis acid. It is an acid with four oxygen atoms, one phosphorus atom, and three hydrogen atoms. Boric acid also goes by the names hydrogen borate, boracic acid, and orthoboric acid. It is a weak acid that is antiviral, antifungal, and antiseptic.
Boric acid is water-soluble and does not have a distinct odour. Under normal circumstances, this chemical occurs as a colourless crystal or as a white powdered substance. Boric acid may be made by combining borax with hydrochloric acid. Wilhelm Homberg was the first one to create boric acid from borax.
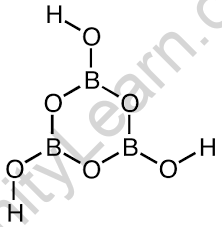
Because of the three oxygen atoms surrounding the boron, it possesses trigonal planar geometry. The length of this bond is 136pm for B-O and 97pm for O-H.
Na2B4O7.10H2O + 2HCl →4B(OH)3 + 2NaCl + 5H2O
B2H6 + 6H2O → 2B(OH)3 + 6H2
BX3 + 3H2O → B(OH)3 + 3HX (X = Cl, Br, I)
Boric acid’s physical properties are as follows:

The following are the chemical properties of boric acid:
H3BO3 → HBO2 + H2O
4HBO2 → H2B4O7 + H2O
H2B4O7→ 2B2O3 + H2O
B(OH)3 + 3ROH → B(OR)3 + 3H2O
B(OH)3 + 6H2SO4→ B(HSO4)4– + 2HSO4– + 3H3O+

In truth, borax and boric acid are the same thing and are commonly used to make homemade laundry soap. Boron is present in all of these materials. Borax is often extracted and purified from tourmaline, kernite, and colemanite. Boric acid is used to extract the mineral sassolite.
Boric acid is a lethal toxin. It is soluble in acetone, water, glycerol, ether, alcohol, methanol, and liquid ammonia, as well as moderately soluble in acetone. Boric acid can be made from borax or by hydrolyzing boron halides or hydrides. Boron oxide crystals are somewhat soluble in cold water and completely soluble in hot water.
Boric acid is also employed as an antiseptic, pesticide, flame retardant, neutron absorber, and precursor in many chemical compounds.