
Courses

By Shailendra Singh
|
Updated on 28 Apr 2025, 12:03 IST
Covalent Bond: Elements having very excessive ionization energies are incapable of shifting electrons and elements having very low electron affinity cannot take up electrons.
The atoms of such factors tend to share their electrons with the atoms of different factors or with different atoms of identical detail in a way that both the atoms acquire octet configuration of their respective valence shell and consequently attain stability. Such association via sharing of electron pairs amongst one of a kind or same types is known as Covalent Bond.
Covalent Bond can be Achieved in Ways:
Also Read: Stoichiometry
If the everyday valence of an atom isn’t happy by way of sharing a single electron pair between atoms, the atoms may additionally proportion multiple electron pairs among them. Some of the homes of covalent bonds are:
Also Check: Concept of Elements, Atoms, and Molecules
All atoms except noble gases have less than 8 electrons in their valence shell. In other phrases, the valence shells of these atoms do no longer have strong configurations. Therefore, they integrate with different atoms to acquire strong electronic configurations.

Therefore, “The tendency of atoms of various elements to attain strong configuration of eight electrons of their valence shells is the purpose of Chemical aggregate” and “The principle of reaching the most of eight electrons within the valence shell of atoms is referred to as octet rule.”
Lewis brought simple symbols to indicate the electrons present within the outer shell of an atom known as the valence electrons. These symbols are called Electron Dot Symbols and the structure of the compound is known as Lewis Dot Structure.
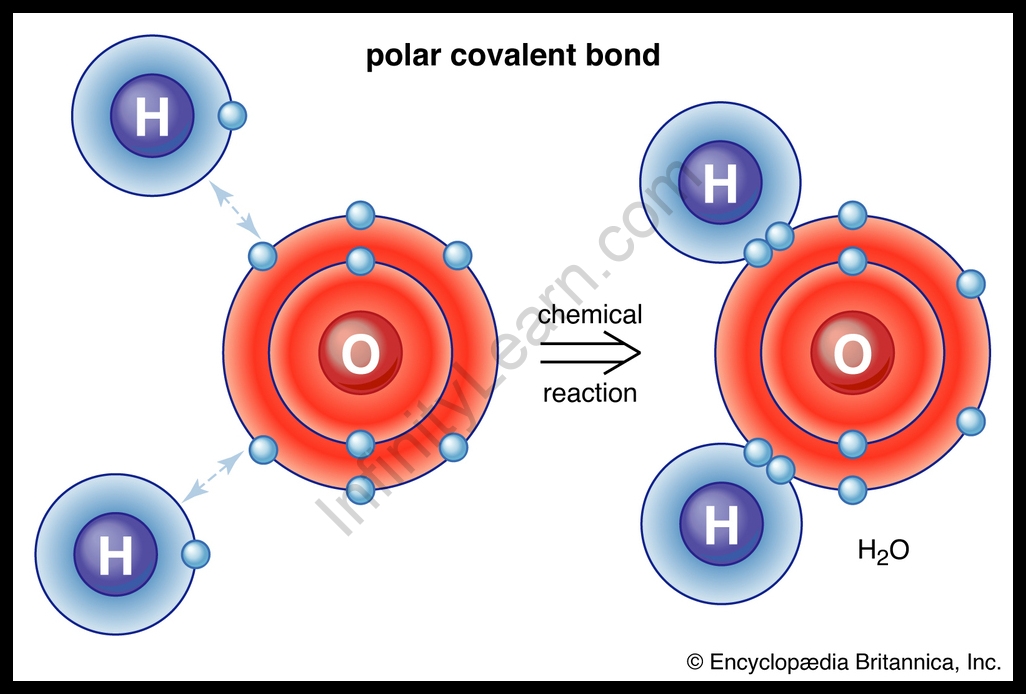
For Example, the oxygen atom which has six electrons in its valence shell completes its octet using sharing its electrons with two hydrogen atoms to shape a water molecule.
Also Check: Percentage Composition
Depending upon the number of shared electron pairs, the covalent bond can be labelled into:
A single bond is shaped when most effectively one pair of electrons is shared between the two participating atoms. It is represented through one sprint (-). Although this form of covalent bond has a smaller density and is weaker than a double and triple bond, it’s miles the most stable.

For Example, the HCL molecule has one Hydrogen atom with one valence electron and one Chlorine atom with seven valence electrons. In this example, a single bond is formed between hydrogen and chlorine by way of sharing one electron.
A double bond is formed while two pairs of electrons are shared among the 2 participating atoms. It is represented with the aid of two dashes (=). Double covalent bonds are much more potent than a single bond, but they’re less solid.
Example: A carbon dioxide molecule has one carbon atom with six valence electrons and two oxygen atoms with 4 valence electrons.
To the whole its octet, carbon stocks two of its valence electrons with one oxygen atom and two with every other oxygen atom. Each oxygen atom shares its electrons with carbon and consequently, there are two double bonds in CO2.
Oxygen-Molecule: In the formation of the oxygen molecule, each oxygen atom has six electrons of its valence shell. Each atom cells for more electrons to complete its octet. Accordingly, the iotas share two electrons each to frame the oxygen particle. Since electron pairs are shared there’s a double bond among the two oxygen atoms.
Ethylene Molecule: In ethylene, each carbon atom shares its valence electron with two hydrogen atoms and the last electrons with the other carbon atom. So there is a twofold connection between the carbon iotas
A triple bond is fashioned while 3 pairs of electrons are shared among the two taking part atoms. Triple covalent bonds are represented through 3 dashes (≡) and are the least stable varieties of covalent bonds.
For Example:
In the formation of a nitrogen molecule, each nitrogen atom having five valence electrons gives 3 electrons to form 3 electron pairs for sharing. Thus, a triple bond is shaped among the two nitrogen atoms.
This form of covalent bond exists wherein the unequal sharing of electrons occurs because of the distinction in the electronegativity of combining atoms. More electronegative atoms may have a stronger pull for electrons. The electronegative distinction among the atoms is more than 0 and less than 2.0. As a result, the shared pair of electrons might be closer to that atom.
For example, molecules form hydrogen bonding as a result of an unbalanced electrostatic capacity. For this situation, the hydrogen particle is associated with electronegative fluorine, hydrogen, or oxygen.
This form of covalent bond is formed on every occasion there’s the same percentage of electrons among atoms. The electronegativity difference among atoms is zero. It takes place wherever the combining atoms have similar electron affinity (diatomic factors).
For example, Nonpolar Covalent Bond is determined in gasoline molecules like Hydrogen fuel, Nitrogen fuel, etc.
It is determined that within the sigma bonds among special atoms, the electron cloud is usually toward the greater electronegative of the two atoms taking part inside the sigma bond. Due to this, there may be an everlasting dipole that arises in the bond, and the covalent bond is stated to be polarized.
A covalent bond is a type of chemical bond where two atoms share one or more pairs of electrons. This typically happens between non-metal atoms. The bond forms because atoms seek to achieve a stable electron configuration, often resembling the nearest noble gas.
Example: In a molecule of water (H₂O), the oxygen atom shares electrons with two hydrogen atoms, forming covalent bonds.
A covalent bond is defined as the electrostatic attraction between the nuclei of two atoms and the shared pair of electrons between them. Covalent bonds form when two atoms, both with a similar tendency to gain electrons, share electrons to complete their outermost electron shell, achieving a more stable electronic configuration.
There are several types of covalent bonds, based on how many pairs of electrons are shared between atoms:
Here are 10 examples of molecules that have covalent bonds:
These molecules demonstrate the variety of ways in which atoms can bond covalently to form stable compounds.