
Courses

The word “electrophile” is a combination of the words “electro,” which means “electronic,” and “phile,” which means “loving.” An electrophile is any molecule, ion, or atom that is electron-deficient in some way. In other words, an electrophile is a chemical that exploits the negative or likes electrons in a molecule. An electrophile has an electron-deficient atom, an empty orbital, or an incomplete octet in the valence shell.
These species comprise molecules with a positive charge or those with a lack of electrons. In a process, an electrophile is a reagent that can receive an electron pair. They often have two fewer electrons than an octet. They attack high electron density regions in the substrate molecule to complete the octet. These are represented by the sign E+.
Lewis acid is electrophilic. Any molecule that can accept a pair of nonbonding electrons, such as the H+ ion, is hence referred to as a Lewis acid.) In other terms, a Lewis acid is an electron-pair acceptor. An electrophile is a substance that accepts a pair of electrons to form a new covalent bond.
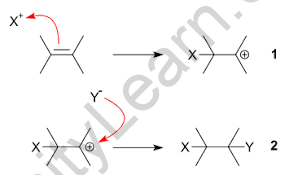
An electrophilic substitution reaction is a chemical reaction in which the functional group of a molecule is replaced by an electrophile.
Electrophilic substitution reactions normally occur in three stages, which are as follows:
Electrophilic substitution reactions inorganic compounds are classified into two types: electrophilic aromatic substitution reactions and electrophilic aliphatic substitution reactions.
In electrophilic aliphatic substitution procedures, an electrophile substitutes the functional group (typically hydrogen) in an aliphatic molecule. These replies can be classified into five types; Halogenation of ketones, Nitrosation, Keto-enol tautomerism, Insertion of a carbene into a carbon-hydrogen bond, Fusion of diazonium (aliphatic).
If the electrophilic attack happens at a 180-degree angle to the leaving group, these electrophilic substitution reactions may result in configuration inversion (attack from the rear).
Aromatic compounds, although having multiple double bonds, do not undergo addition reactions. The high stability of the ring systems produced by full electron delocalization accounts for their lack of reactivity to addition processes (resonance).

Aromatic compounds respond by electrophilic aromatic substitution reactions that preserve the aromaticity of the ring system. For example, bromobenzene is formed when benzene reacts with bromine.
In electrophilic aromatic substitution operations, an atom connected to an aromatic ring is replaced with an electrophile. Aromatic nitrations, aromatic sulphonation, and Friedel-Crafts reactions are included in this type of reaction.
It is critical to emphasize that the aromaticity of the aromatic component is preserved in electrophilic aromatic replacements. As a result, aromatic rings and iodine, bromine, or chlorine can be used to generate aryl halides in these methods.
Anhydrous aluminium chloride is a very helpful Lewis acid in the production of electrophiles from aromatic ring chlorination, alkylation, and acylation. Electrophiles are formed in the presence of Lewis acid. The electron pair from the attacking reagent is accepted by the Lewis acid. In that sequence, the electrophiles generated are Cl+, R+, and RC+O. (from the combination of anhydrous aluminium chloride and the attacking reagent).
By creating a sigma complex or an arenium ion, the electrophile attacks the aromatic ring. One of the hybridized carbons in this uranium ion is sp3. In a resonance configuration, this arenium ion finds stability. The sigma complex or arenium ion loses its aromatic property because electron delocalization terminates at the sp3 hybridized carbon.
Finally, the proton is ejected from the complex of arenium or sigma ions. This happens when a reagent like AlCl4 comes into contact with the sp3 hybridized carbon. As a consequence, the molecule’s aromatic character is restored, and the electrophile finally takes the place of the aromatic component’s hydrogen.
Many electrophiles are not electrophilic enough to react on their own, or the reaction takes a long time. We may also speed up the electrophilic substitution process by using Lewis acids. In the presence of AlCl3 and FeCl3, for example, the electrophilic substitution of benzene with Cl2 can happen at a much faster rate.
When an electrophile replaces the hydrogen atom of benzene, the process is known as electrophilic substitution. These reactions are particularly spontaneous because the aromaticity of benzene is not changed throughout the process. Nitration, sulfonation, halogenation, Friedel Craft’s alkylation, acylation, and other basic benzene electrophilic substitution reactions.
Catalysts like Iron (Fe) or Aluminum Chloride (AlCl3) are used in aromatic ring chlorination and bromination to enhance the reaction rate and direct the substitution to the desired position on the aromatic ring.
Nucleophilic substitution is when a nucleophile replaces a group in a molecule, while electrophilic substitution is when an electrophile replaces a group. Both reactions change the molecule while keeping the core structure intact.
The five types of electrophilic substitution reactions are halogenation, nitration, sulfonation, Friedel-Crafts alkylation, and Friedel-Crafts acylation. They involve replacing a hydrogen atom on an aromatic ring with different groups.
An example of an electrophile is a bromine ion (Br+), which can seek out electrons to form bonds, typically with bases.
Nucleophilic substitution reaction refers to a reaction where a nucleophile, an atom or molecule with extra electrons, replaces a leaving group in another molecule.
There are two main types of nucleophilic substitution reactions: SN1, where the substitution happens in two steps, and SN2, where the substitution happens in one step.
Nucleophilic substitution can be either SN1 or SN2, depending on the mechanism. SN1 is a two-step process and SN2 is a one-step process. Both result in a nucleophile replacing a leaving group in a molecule.