
Courses

By Shailendra Singh
|
Updated on 23 Jan 2025, 14:35 IST
The overlapping of atomic orbitals is a fundamental concept that distinguishes sigma (σ) and pi (π) bonds from other types of covalent bonds. This overlapping is the primary mechanism through which covalent bonds are formed. While sigma bonds are created by the head-on overlap of atomic orbitals, pi bonds are formed by the lateral or sideways overlap of atomic orbitals.
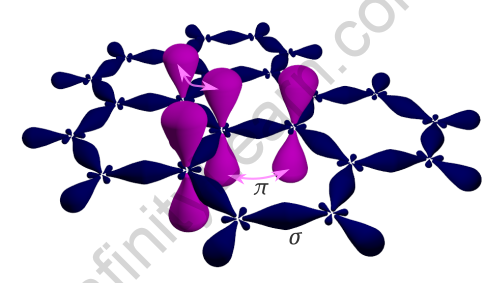
A sigma bond is a type of covalent bond formed by the direct, head-on overlap of atomic orbitals along the internuclear axis. Due to this direct overlap, sigma bonds are considered the strongest type of covalent bonds. The electrons involved in sigma bonds are localized between the nuclei of the bonding atoms. Typically, every single bond in a molecule is a sigma bond.
| Type of Overlap | Example | Description |
| S-S Overlap | H₂ molecule | Head-on overlap of 1s orbitals of hydrogen atoms. |
| S-P Overlap | NH₃ molecule | Overlap of 1s orbital of hydrogen with 2p orbital of nitrogen. |
| P-P Overlap | Cl₂ molecule | Head-on overlap of 3p orbitals of chlorine atoms. |
Pi bonds are formed by the lateral or sideways overlap of atomic orbitals that are parallel to each other. During pi bond formation, the axes of the overlapping orbitals remain parallel, and the overlap occurs perpendicular to the internuclear axis. Due to the lesser degree of overlapping, pi bonds are weaker than sigma bonds.
| Type of Bond | Example | Description |
| Single Bond | H₂ molecule | Contains only a sigma bond. |
| Double Bond | Ethene (C₂H₄) | Contains one sigma bond and one pi bond. |
| Triple Bond | Acetylene (C₂H₂) | Contains one sigma bond and two pi bonds. |
| Aspect | Sigma Bond | Pi Bond |
| Formation | Head-on overlap of atomic orbitals. | Sideways overlap of atomic orbitals. |
| Strength | Stronger due to greater overlap. | Weaker due to lesser overlap. |
| Electron Cloud | Symmetrical along the internuclear axis. | Above and below the plane of the bonded atoms. |
| Flexibility | Allows free rotation of bonded atoms. | Restricts rotation of bonded atoms. |
| Occurrence | Found in single, double, and triple bonds. | Found only in double and triple bonds. |
| Overlap | Involves s-s, s-p, or p-p overlap. | Involves only p-p overlap. |
Understanding sigma and pi bonds is crucial for mastering molecular structure, bonding, and reactivity, especially for competitive exams like JEE. These concepts form the foundation for topics such as hybridization, resonance, and molecular orbital theory. Sigma and pi bonds play a vital role in:
Students preparing for competitive exams should focus on mastering the characteristics, formation, and examples of sigma and pi bonds. This understanding not only helps in exams but also lays the groundwork for advanced studies in chemistry.


Two pi bonds and one sigma bond make up a triple bond. Similarly, one double bond is made up of one sigma bond and one pi bond. Sigma bond has always been formed by single bond.
The benzene ring has been made up of six carbon-carbon single bonds that are all sigma bonds. Also, there are six carbon-hydrogen sigma bond. As a result, a benzene molecule has a total of 12 sigma bonds. The aromatic is distinguished by alternating double bonds between carbon atoms. As a result, a benzene molecule has a total of three pi bonds.