
Courses

By Shailendra Singh
|
Updated on 20 Jan 2025, 18:49 IST
Carbocation rearrangements are prevalent in organic chemistry and are described as the transition of a carbocation from an unstable to a more stable state via different structural reorganizational “shifts” within the molecule.
When an alkyl halide, alcohol, or alkene is converted into a carbocation, it may undergo rearrangement. Carbocation rearrangements are classified into two types: hydride shifts and alkyl shifts. When the ensuing carbocation is rearranged, it will react further to generate a final product with a different alkyl skeleton than the initial material.
Also Check: Bohr's Theory of Hydrogen Atoms
Any reaction that involves a carbocation intermediate is susceptible to rearrangement. During some electrophilic additions, carbocation rearrangements may occur.


No rearrangement may occur immediately after the synthesis of the tertiary carbocation which will make it more stable.
An alkyl shift occurs when a carbocation lacks a hydrogen atom that is present on the neighbouring carbon atom and is easily accessible or present to undergo a rearrangement process. It is also possible that the hybrid shift is unable to produce a stable carbocation. In this scenario, an alkyl shift is used.
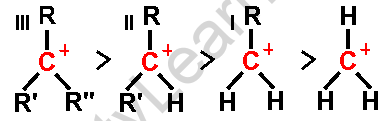
Rearrangement occurs to form a more stable carbocation. This stability is achieved by shifting alkyl groups or hydrogen atoms through hydride or alkyl shifts, resulting in tertiary carbocations being more stable than secondary or primary ones.
The two most common rearrangements are hydride shifts (movement of a hydrogen atom with its bonding electrons) and alkyl shifts (movement of an alkyl group). Both aim to transform less stable carbocations into more stable ones.