
Questions
Conversion of water to steam is accompanied by which process?
Adiabatic
Isothermal
Isochoric
Cyclic
detailed solution
Correct option is B
First, we'll see what happens when water is converted to steam; that is a liquid state is converted to a gaseous state. When heat is continuously provided to the water its temperature starts to rise with time, up to a specific temperature. then temperature, when further heat is provided the heat energy, is used up for breaking the intermolecular bonds in the liquid state and not for increasing the kinetic energy. Therefore the temperature remains constant whenever a change in phase occurs either from liquid to gas that's the boiling Point or from solid to liquid that is the melting point as shown in figure 1.
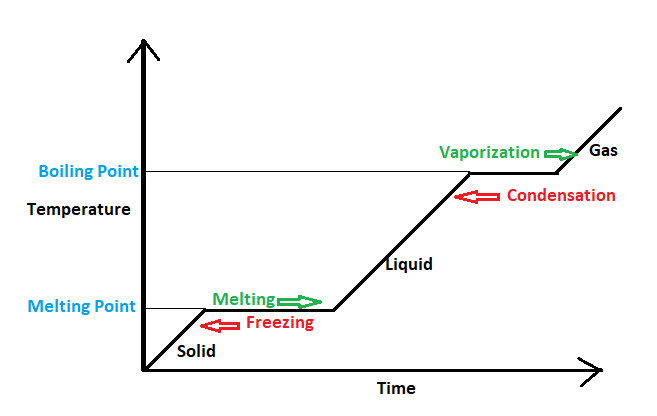
Figure 1
These sorts of Thermodynamic processes which take place with constant temperatures are known as Isothermal processes. So conversion of water into steam is an Isothermal procedure. hence, option B is the right answer.
we'll also discuss the definition of the other terms given in the question as it will be helpful in future
A. An natural process takes place without transferring heat or mass between a thermodynamic system and its surroundings.
C. An isochoric process may be a thermodynamic process in which the volume of the enclosed system undergoing such a process remains unchanged.
D. A cyclic process may be a thermodynamic process where the starting and ending state is the same.
Hence, the correct answer is option B.
Talk to our academic expert!
Practice More Questions
Easy Questions
Moderate Questions
Difficult Questions


799 666 8865
support@infinitylearn.com
6th Floor, NCC Building, Durgamma Cheruvu Road, Vittal Rao Nagar, HITEC City, Hyderabad, Telangana 500081.
free study material
JEE Mock Tests
JEE study guide
JEE Revision Notes
JEE Important Questions
JEE Sample Papers
JEE Previous Year's Papers
NEET previous year’s papers
NEET important questions
NEET sample papers
NEET revision notes
NEET study guide
NEET mock tests



