
Organic chemistry stands out due to its three-dimensional nature, which profoundly influences the properties and behavior of molecules. One of the most fascinating aspects of organic chemistry is the concept of isomerism, where molecules with the same molecular formula can exist in different structural arrangements. These variations lead to differences in physical and chemical properties. Isomers are molecules that share the same molecular formula but differ in the arrangement of their atoms, which can significantly alter their characteristics.
Structural isomerism, also known as constitutional isomerism according to the International Union of Pure and Applied Chemistry (IUPAC), refers to isomers that differ in the connectivity of their atoms rather than their spatial arrangement. Despite having the same molecular formula, these compounds exhibit distinct chemical and physical properties, making structural isomerism a crucial concept in organic chemistry.
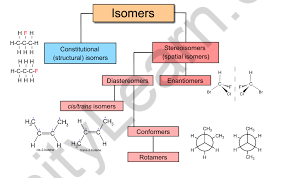
Structural isomers share the same molecular formula but differ in how their atoms are connected. These differences can result in molecules having:
Structural isomerism is particularly significant in hydrocarbons like alkanes, where increased chain length leads to a larger number of possible isomers. For example, butane (C4H10) exists as two isomers: n-butane and isobutane. As the number of carbon atoms in an alkane increases, the number of possible structural isomers rises exponentially.
Structural isomerism is broadly classified into three types:
This type occurs when compounds differ in the arrangement of their carbon skeletons. Chain isomers have the same molecular formula but vary in the branching of the carbon chain.
Example:
| Molecular Formula | Isomer Name | Structure |
| C4H10 | n-Butane | CH3-CH2-CH2-CH3 |
| C4H10 | Isobutane | (CH3)2CH-CH3 |
In position isomerism, compounds differ in the location of a substituent, functional group, or unsaturation in the molecule.

Example:
| Molecular Formula | Isomer Name | Structure |
| C3H8O | 1-Propanol | CH3-CH2-CH2OH |
| C3H8O | 2-Propanol | CH3-CHOH-CH3 |
Functional group isomerism arises when compounds have the same molecular formula but belong to different functional groups.
Example:
| Molecular Formula | Isomer Name | Functional Group | Structure |
| C3H6O | Propanal | Aldehyde | CH3-CH2-CHO |
| C3H6O | Acetone | Ketone | CH3-CO-CH3 |
| Property | Chain Isomerism | Position Isomerism | Functional Group Isomerism |
| Molecular Formula | Same | Same | Same |
| Atom Connectivity | Different carbon skeleton | Same skeleton, different group position | Different functional groups |
| Physical Properties | Often different (boiling/melting points) | May vary slightly | Typically distinct |
| Chemical Properties | May differ significantly | Generally similar | Markedly different |
Structural isomerism has significant implications in both academic and applied chemistry:
Structural isomerism is a cornerstone of organic chemistry, offering insight into the diversity and complexity of molecular structures. By understanding how atoms can be arranged differently, chemists can predict and manipulate properties to suit various applications, from pharmaceuticals to industrial processes. The study of structural isomers underscores the importance of molecular structure in determining the behavior and utility of compounds.
The bonding patterns of structural isomers can be used to identify them. The atoms in the compound are the same, but they are linked in such a way that they form different functional groups.
Structural isomers are compounds that have the same molecular formula but have distinct atom arrangements or different bonds. Isobutane and butane, for example, contain the same amount of hydrogen and carbon atoms and so have the same chemical formula.