




















Courses
Q.
When liquid ′A′ is treated with a freshly prepared ammonical silver nitrate solution, it gives a bright silver mirror. The liquid forms a white crystalline solid on treatment with sodium hydrogen sulphite. Liquid ′B′ also forms a white crystalline solid with sodium hydrogen sulphite, but it does not give a test with ammoniacal silver nitrate. Which of the two liquids is aldehyde? Write the chemical equations of these reactions also.
(a) Identify (A), (B) and (C).
(b) Write the structures (A), (B) and (C).
Write the reactions of compound (A) and (B)[Aldehydes, Ketones and Carboxylic acids]
OR
Arrange the following compounds un increasing order of their property as indicated:
(i) Acetaldehyde, acetone, Di – tert – butyl ketone, Methyl tert – butyl ketone
( reactivity towards HCN)
(ii)
( acid strength)
(iii) Benzoic acid , 4 – Nitrobenzonic acid, 3, 4 – Dinitrobenzoic acid
4 – Methoxybenzoic acid ( acid strength)
see full answer
Your Exam Success, Personally Taken Care Of
answer is 1.
(Unlock A.I Detailed Solution for FREE)
Best Courses for You

JEE

NEET

Foundation JEE

Foundation NEET

CBSE
Detailed Solution
Liquid ‘A’ gives both a test and forms a silver mirror when treated with freshly prepared ammonium silver nitrate solution and it forms white crystalline solid with sodium hydrogen sulphite. Now liquid ‘A’ must be an aldehyde because ketones does not give a silver mirror test. let’s see the reactions
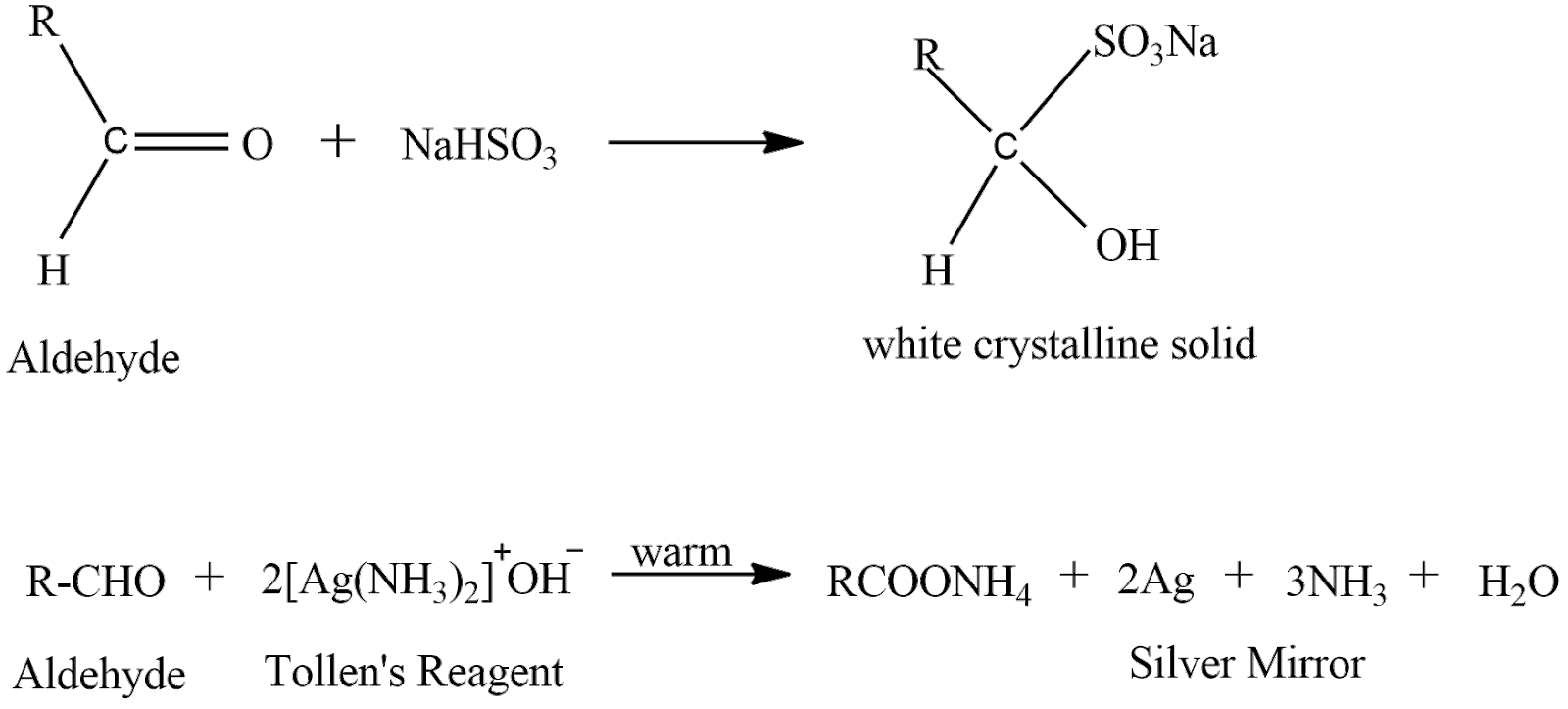
In the first reaction aldehyde reacts with sodium hydrogen sulphite to give a complex product which is a white colored crystalline solid. In the second reaction aldehyde reacts with ammoniacal silver nitrate solution to give a silver mirror.
Liquid ‘B’ forms white crystalline solid when treated with sodium hydrogen sulphite but does not give silver mirror test, this signifies that liquid ‘B’ must be a ketone because ketone does not give silver mirror test. Here are the reactions
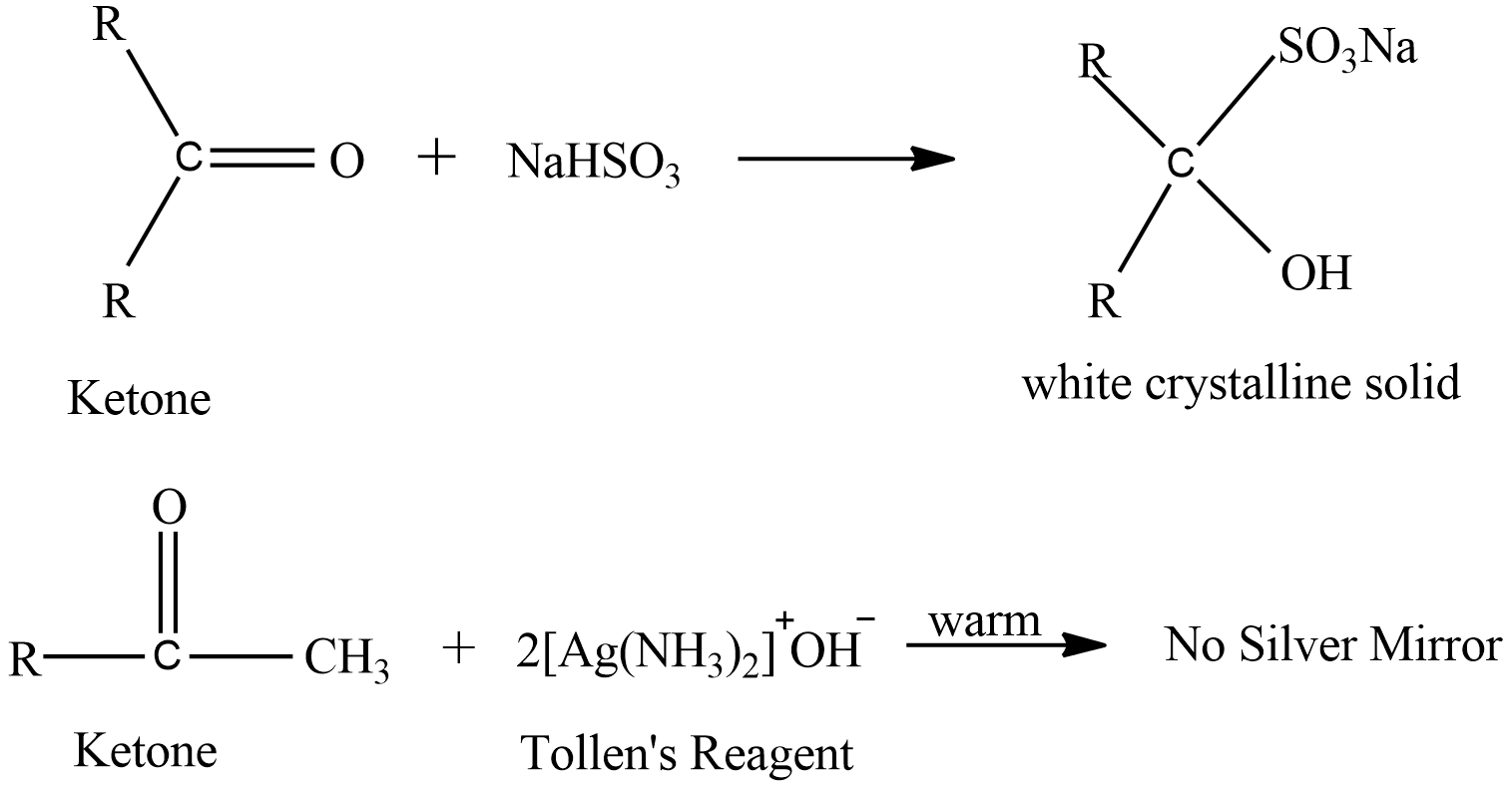
OR
(i) In the given compound, the +I effect increase hence reactivity of HCN towards these compound decreases thus, the order is, Di-tert-butyl ketone < Methyl tert-butyl ketone < Acetone < Acetaldehyde.
(ii) CH3CH2CH(Br)COOHCH3CH(Br)CH2COOH (CH3)2COOH
(iii)Since, electron donating groups decreases the acid strength, therefore, 4-methoxy benzoic acid is a weaker acid than benzoic acid. Further, since electron-withdrawing groups increase the acid strength, therefore, both 4-nitrobenzoic acid and 3, 4-dinitrobenzoic acid are stronger acid than benzoic acid.
Further, due to presence of an additional NO2 at m-position with respect to COOH group, 3, 4-dinitrobenzoic acid thus the overall acid strength increase in the order:
4-methoxy benzoic acid < benzoic acid < 4-nitrobenzoic acid < 3, 4-dinitrobenzoic acid.


courses
No courses found
Ready to Test Your Skills?
Check your Performance Today with our Free Mock Test used by Toppers!
Take Free Test



