Osmosis in Biology
The process by which solvent atoms pass a low concentration solution into a high concentration solution in a water-soluble membrane. It is an ongoing process that takes place without the use of any energy costs. The transport of solvent atoms continues from low to high until the concentration on both sides of the membrane is equal.
Wilhelm Pfeffer, a German botanist, was the first to investigate this process in depth in 1877. Previous research on leaky membranes (e.g., one of the animals) and the transport of escape water and chemicals was less accurate. The British scientist Thomas Graham coined the term osmose (now osmosis) in 1854.
When the solution is separated from the pure solvent by a membrane that can penetrate into the solvent but not into the solute, the solution will usually dissolve as the solvent is absorbed into the membrane. By increasing the osmotic pressure of the liquid by a given amount, this process can be reduced.
In 1886, the Dutch-born chemist Jacobus Henricus van ‘t Hoff showed that if the solute was reduced to such an extent that the partial pressure of the vapour over the solution complied with Henry’s law (i.e., equal to its concentration in solution), then the pressure of -osmotic varies. by concentration and temperature in the same way as a gas taking the same volume. This interaction led to the development of formulas to measure the molecular weights of solvents in soluble solutions based on solvent cooling, boiling, and vapour pressure.
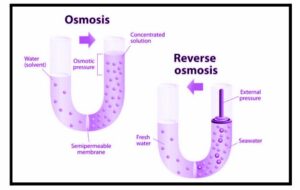
Osmotic Pressure
The osmotic pressure is the minimum pressure required to prevent the internal flow of pure solvent solution into an easily accessible barrier. It can also be described as a measure of the availability of a solution to absorb pure solvent by osmosis. The highest osmotic pressure that can develop in solution if removed from its pure solvent by a flawless membrane is known as potential osmotic pressure.
When two solutions with different amounts of solute are separated by a penetrating membrane, osmosis occurs. From low concentration to a solution with increasing solute concentration, solvent molecules pass through the membrane with great popularity. The flow of solvent atoms will continue until equilibrium is reached.
What is a Semipermeable Membrane?
It is a small barrier between two solutions that allow certain components of the solution, usually a solvent, to pass through. A waterproof membrane is a barrier that allows some molecules to fall but prevents others from doing so. A slightly accessible barrier acts as a filter actually. Membranes that can move the water of different kinds can block molecules of different sizes. Biological or synthetic materials can be used to form a flawless membrane.
A membrane that can be penetrated or the membrane may not be easily penetrated in other words.
Types of Osmosis
Generally, there are two types of osmosis. These are

Endosmosis
When a cell is immersed in a hypotonic solution, the fluid that runs inside the cell causes it to swell or form plasmolyze. This is because the solute concentration of the solution is less than the concentration inside the cell. This process is known as endosmosis. The osmosis facing the inside of a cell or vessel is known as endosmosis. It occurs when the water-energy outside the cell is higher than the water energy inside the cell. As a result, the solute concentration of the surrounding solution is less than that of the cytoplasm. Hypotonic solutions are the name of this type of solution. In endosmosis, water molecules pass through the cell membrane and inside the cell. The flow of water through the cells causes them to swell.
Example: Dried swells when immersed in ordinary water.
Exosmosis
When a cell is immersed in a hypertonic solution, the water inside the cell flows out, and thus the plasmolysis of the cell (becomes flaccid). This is because solute concentration in solution is more than concentrated within the cytoplasm. This process is known as exosmosis. Exosmosis is osmosis of a cell or outer vessel. Occurs when the water outside the cell is less than the water inside the cell. As a result, the concentration of the surrounding solution is higher than that of the cytoplasm. Hypertonic Solutions is the name of these types of solutions. Exosmosis is the movement of water molecules from a cell across the cell membrane. The migration of water from cells causes the cells to shrink.
Example: Dry placed in a concentrated salt mass shrinks.
Reverse Osmosis
It is a separation process that uses pressure to force a solvent through a semipermeable membrane that holds the solute on one side and makes the solvent pass on the other side. It uses pressure to force the solvent to move from the molten surface of the surface to a soft lower concentration. Thus variable osmosis can be termed as opposed to normal osmosis.
Forward Osmosis
It is natural to use a semi-permeable membrane to separate soluble solutes in water. The previous osmosis process is beneficial to a variety of industrial water purification systems, including wastewater management, product concentration, and recycling of water, thanks to a highly efficient filtering system, which ensures that only clean water is available in the feed solution. It uses less energy than other hydraulic pressure systems as it relies on the natural forces of osmotic pressure.
Application: desalination, purification of wastewater, production of osmotic energy.
Osmotic Concentration
The osmolarity of the solution is a measure of how concentrated the solute is within one litre of solution. Osmoles (Osm) are used to measure osmotic concentration, represented as osmoles per litre (Osm / L). In some cases, osmotic concentrations are also expressed in millimoles per litre (mmol / L). The osmotic concentration of the solute increases as the amount of water or solvent decreases. Similarly, increasing the amount of solvent in the solution reduces the solute concentration of the osmotic.
Isotonic Solution
In an isotonic solution, the cell is parallel to the surrounding environment where the concentration of solvents internally and externally is the same (the eye means equal in Latin). There is no concentration gradient in this case, which means that no water movement is necessary to enter or exit. However, water molecules are free to enter and leave the cell at the same speed in both directions.
Hypotonic Solution
The concentration of soluble in hypotonic fluid is lower than inside the cell (the origin of hypo is Latin less or less). Water enters the cell due to the concentration difference between the compartments. Animal cells are less tolerant of this condition than plant cells. Water flows into the intercellular space as a large central vacuole fills the plants with water. Turgor pressure is caused by a combination of these two actions, which push through the cell wall, causing it to protrude. The cell wall acts as a barrier to prevent the cell from exploding. An animal cell, on the other hand, will grow until it explodes and dies if left in a very hypertonic solution.
Hypertonic Solution
The prefix hyper indicates “up” or “up” in Latin. The concentration of soluble in a hypertonic fluid is higher than inside the cell. Water leaks out quickly, causing the cell to shrink or shrink. This is seen in red blood cells undergoing a process known as crenation. Due to internal factors, plant cells in a hypertonic solution may resemble pincushions. The cell membrane emerges from the cell wall, but in the plasmodesmata, it remains connected. Plasmodesmata are small tubes that transport and communicate information between plant cells. Plasmolysis occurs when the inner lining thickens, making the plasmodesmata stronger.
Osmosis in Plant Cell
When a cell is immersed in a hypertonic solution that is more concentrated than a cell, it will shrink due to water loss and eventually die. For example, if a piece of carrot was placed in a mixture of salt water it would soften and become fragmented as the cells would shrink. Conversely, when a piece of carrot is soaked in an isotonic solution, it can swell and expand. Normally, a normal cell can explode, but a strong cell wall in a carrot cell protects it from explosion. As water enters the cell, it expands, until it creates greater pressure on the cell wall to expand further. However, the cell wall slows down with equal force and no more water can enter.
Osmosis plays an important role in the transport of water to plants. Solute concentrations increase as they move from the soil to the root cells and to the leaf cells. The pressure difference helps to push the water upwards. Osmosis also controls the evaporation of leaves by controlling the size of the stomata in place of the leaves.
Significance of Osmosis
Plants
- Osmosis helps maintain the water content within the plant cell
- It provides turgidity to the soft cells of the plant body.
- Osmosis controls the absorption of water through the hair follicles from the ground.
- It controls the flow of water from xylem objects to nearby cells
- High osmotic pressure provides plant resistance against drought damage
In animals
- Osmosis controls the flow of solvents, liquids, and gases throughout cells.
- The semi-permeable membrane that selects the cell selectively allows substances to pass through the cell. This helps in the removal of toxic metabolic waste such as urea.
- Osmosis also helps to absorb fluid from the intestines into the bloodstream.
Significance of Osmosis
- Osmosis plays an important role in the transport of nutrients and the release of metabolic waste within the living cell.
- Strengthens the internal movement of water and intracellular fluid levels within the cell.
- Osmosis also controls the cell-to-cell distribution and maintains the mechanical structure of the cell.
- In plants, the tips of growing roots remain turgid and can easily penetrate the soil due to osmosis.
- Osmosis plays a major role in seed germination.
Examples of Osmosis
- Absorption of water by plant roots in the soil.
- The protective cells of the plant cell are affected by osmosis. When a plant cell is full of water the guard cells swell up so that the stomata open and release more water.
- If you keep your fingers in the water for a long time, they become prunes. The reason for this is that the skin absorbs water and thickens.
FAQs on Osmotic Pressure
What is osmotic pressure?
It is the amount of pressure needed to prevent water from separating the membrane through osmosis. The concentration of the solute in the solution determines the amount of pressure. It can be calculated using the number below.
Π = MRT
When Π indicates osmotic pressure
R fixed gas
M refers to the molar concentration of the solute
T temperature
Osmotic pressure plays a role in the transport of solutes from cell entry and exit. It also prevents the flow of water inside the immovable membrane and causes osmosis.
In water, thermal conductivity refers to the transfer of thermal energy from the upper to the lower extremities. The action of a nuclear reactor creates the need for the distribution of neutrons with a substance that normally disperses neutrons but rarely absorbs them.
Examples of Dispersion
A drop of food colouring spreads all over the water in the glass.
When you put a sugar cube in water it will eventually melt and the water will taste better.
What are the hypertonic, hypotonic, and isotonic solutions?
Hypertonic Solutions: It is a type of solution with a higher volume of solute than any other solution. Hypertonic solutions have high osmotic pressure.
Example: 5% sugar solution and 0.45% salt.
Hypotonic Solution: It is a type of solution with a lower solute concentration than any other solution. Hypotonic solutions have low osmotic pressure.
Example: 0.45% saline solution. Hypotonic solutions are used to treat patients with diabetic ketoacidosis
Isotonic Solutions: Two solutions with the same combination of soluble particles and the same osmotic pressure.
Example: 0.9% of normal saline mixture, lactation rings (sterile solution composed of Sodium Chloride, Sodium Lactate, Potassium Chloride, and Calcium Chloride)
