
P Block Elements: The final electron in a p-block element enters the outermost p orbital. Because there are three p orbitals, the maximum number of electrons that can be accommodated in a set of p orbitals is six. As a result, there are six groups of p–block elements in the periodic table, numbered 13 to 18. The groups are led by boron, carbon, nitrogen, oxygen, fluorine, and helium. Their electronic valence shell configuration is ns2np1-6 (except for He). The inner core of the electronic configuration, on the other hand, may differ. The difference in the inner core of elements has a significant impact on their physical properties (such as atomic and ionic radii, ionization enthalpy, and so on), as well as their chemical properties.
As a result, there is a lot of variation in the properties of elements in a group of p-blocks. A p-block element’s maximum oxidation state is equal to the total number of valence electrons (i.e., the sum of the sand p-electrons). Clearly, as one moves to the right of the periodic table, the number of possible oxidation states grows. In addition to this so-called group oxidation state, p-block element can have other oxidation states that differ by a unit of two from the total number of valence electrons, but this is not always the case.
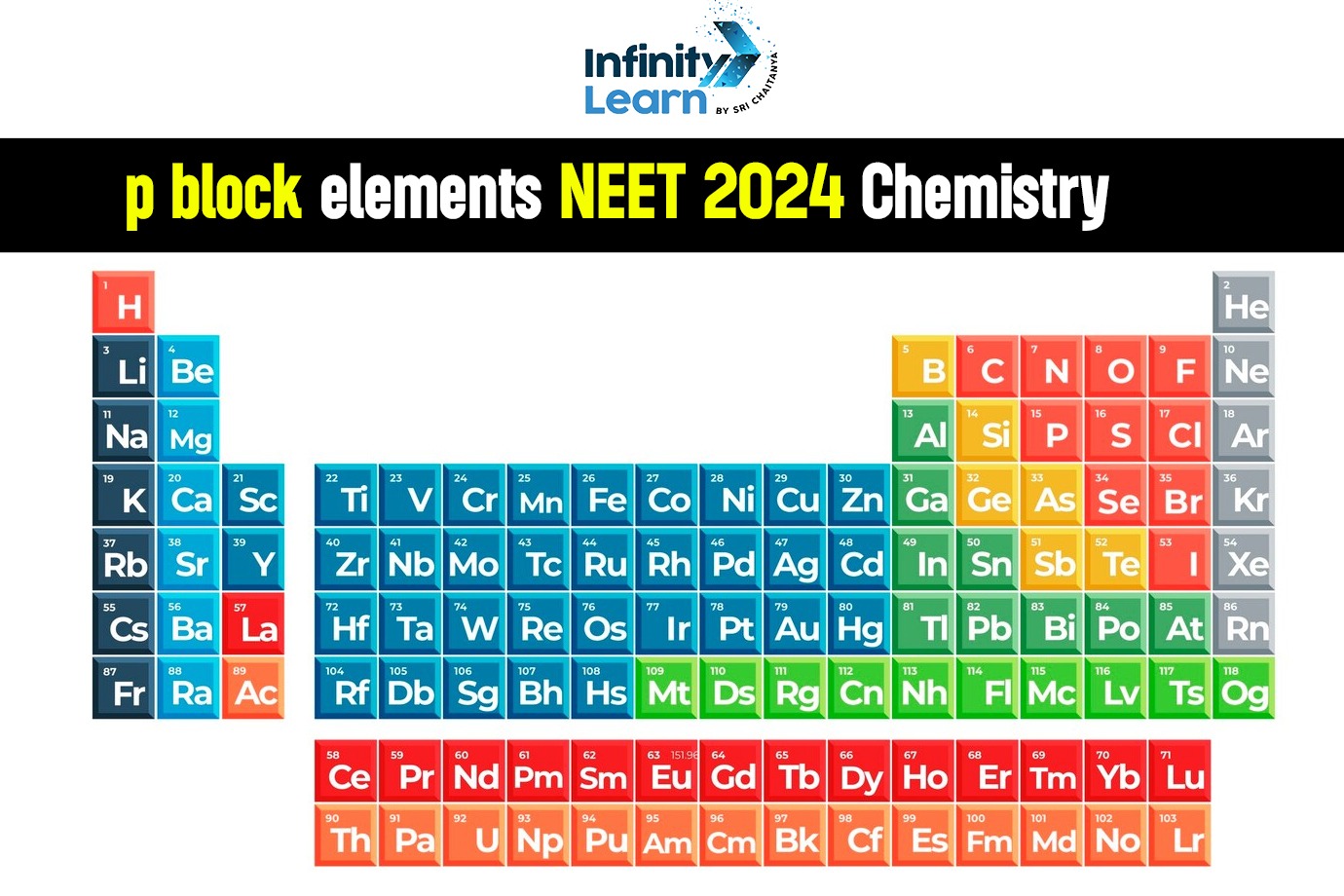
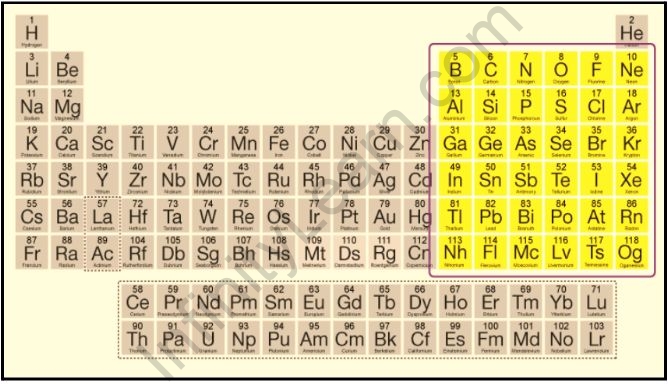
The last electron in a p-block element occupies a p-orbital, and they are found in groups 13, 14, 15, 16, and 17. (except helium). P-block elements are classified into six groups because p-block orbitals can only hold a maximum of six electrons. Each group of p-block elements is identified by the first element in the group. The Boron Family is known as Group 13, the Carbon Family is known as Group 14, the Nitrogen Family is known as Group 15, the Oxygen Family is known as Group 16, the Halogen or Fluorine Family is known as Group 17, and the Noble Gas Family or Neon Family is known as Group 18. Helium is not a p-block element, but it is a noble gas, so it is included in the noble gas family. Helium is an s-block element.
The last electron in a P block element can enter any of the three p-orbitals of its respective shell. Because a p-subshell has three degenerate p-orbitals, each of which can accommodate two electrons, there are six groups of p-block elements in total. Because of their tendency to lose an electron, these elements are shiny and usually a good conductor of electricity and heat. In a P-block element like gallium, you will discover some amazing properties of elements. It’s a metal that can melt in your hand. Because silicon is an important component of glass, it is also one of the most important metalloids of the p-block group. P-block elements are found in groups 13, 14, 15, 16, and 17 and have the last electron occupying p-orbitals (except helium).
P-block elements are divided into six groups because p-block orbitals can only hold a maximum of six electrons. Each group of p-block items is identified by the first member. The Boron Family is known as Group 13, the Carbon Family is known as Group 14, the Nitrogen Family is known as Group 15, the Oxygen Family is known as Group 16, the Halogen or Fluorine Family is known as Group 17, and the Noble Gas Family or Neon Family is known as Group 18. It is important to remember that helium is not a p-block element. These elements include certain metals, all nonmetals, and metalloids.
S-block and p-block elements are joined to form normal or representative elements (except zero group elements). Each periodic table period ends with a noble gas with a closed shell ns2np6 structure, which is a member of the zero groups (18th group). There are two chemically relevant nonmetal groups before the noble gas group. Halogens (group 17) and chalcogens (group 18) are the two types (group 16).
The element in which the last electron enters the outermost p-subshell is referred to as a P block element. In the periodic table, the P block begins with the 13th group and ends with the 18th group.

 When the total number of valence electrons, i.e. the sum of S and P electrons, is equal to a total number of valence electrons, the oxidation state of elements in the p – block is maximum. One of the most intriguing aspects of the p-block elements is the presence of both nonmetals and metalloids.
When the total number of valence electrons, i.e. the sum of S and P electrons, is equal to a total number of valence electrons, the oxidation state of elements in the p – block is maximum. One of the most intriguing aspects of the p-block elements is the presence of both nonmetals and metalloids.
The typical orbital of each element block is determined by the electrons with the highest energy.:
S-block metals: The first two groups of the periodic table, the s-block metals are as follows:
Elements of the P-block:
D-block elements are transition metals from element groups 3 through 12. Elements of a D-Block:
F-block: Inner transition elements, typically from the lanthanide and actinide series, which include lanthanum and actinium. These are metals with the following properties:
P-block elements are the set of elements found in groups 13 to 18 of the periodic table, excluding helium. These elements have their outermost electrons located in the p-orbital. There are 35 p-block elements in total, and they exhibit a broad range of physical and chemical properties, including metals, metalloids, and non-metals. Elements like nitrogen, oxygen, and fluorine are part of this block, known for their roles in various chemical reactions and applications.
The term p-block refers to the placement of the elements’ valence electrons in the p-orbital. In the periodic table, elements are categorized into blocks based on their electron configurations, and the p-block specifically includes elements whose outermost electrons are found in the p-subshell. This differentiates them from elements in other blocks like the s-block, d-block, and f-block, where valence electrons occupy s, d, and f orbitals, respectively. These elements are considered part of the main group due to their standard, predictable properties.
Yes, the p-block elements are part of the NEET 2024 syllabus. Despite some syllabus reductions, this crucial chapter remains included due to its significance in both Class 11 and Class 12 NCERT chemistry.
Based on previous trends, you can expect about 2-3 questions from the p-block chapter, contributing to roughly 7% of the total chemistry section in NEET .