Table of Contents
Introduction
Glucose is a simple sugar and one of the most important carbohydrates found in nature. It is also known as dextrose or blood sugar. Glucose is a primary source of energy for living organisms, including humans.
Chemically, glucose belongs to the monosaccharide group, which means it is composed of a single sugar unit. It has a molecular formula of C6H12O6, indicating that it consists of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Overall, glucose is a fundamental molecule in biology and serves as a critical energy source for organisms. Its widespread presence and vital role in various biological processes make it a key component in the study of nutrition, metabolism, and human health.
Structural Formula of Glucose
In this structure, each carbon atom is represented by a C, and each hydrogen atom is represented by an H. The hydroxyl groups (OH) are indicated by the branches extending from the carbon atoms, except for the carbon atom on the far right, which has a hydrogen atom (H) attached to it.
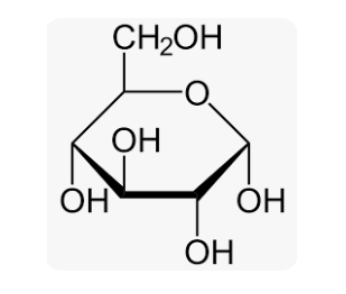
Physical Properties of Glucose
- State: At room temperature, glucose exists as a white crystalline solid. It is often seen in the form of fine granules or powder.
- Solubility: Glucose is highly soluble in water. It readily dissolves in water to form a clear, colorless solution. It exhibits a high solubility due to the presence of multiple hydroxyl (OH) groups in its structure, which can form hydrogen bonds with water molecules.
- Melting Point: Glucose has a relatively high melting point compared to other sugars. It melts at around 146 to 150 degrees Celsius (295 to 302 degrees Fahrenheit).
- Sweetness: Glucose has a sweet taste, although it is less sweet compared to other sugars such as fructose or sucrose.
- Optical Activity: Glucose is optically active, meaning it can rotate the plane of polarized light. It is dextrorotatory, meaning it rotates polarized light to the right (clockwise) when observed through a polarimeter.
- Hygroscopicity: Glucose has hygroscopic properties, which means it has a tendency to absorb moisture from the surrounding environment. It can readily attract and retain water molecules.
- Stability: Glucose is relatively stable under normal conditions. It does not undergo significant decomposition or degradation at room temperature. However, it can undergo Maillard reactions, a non-enzymatic browning reaction, when heated in the presence of amino acids.
Also Check
Chemical Properties of Glucose
- Reactivity with Oxidizing Agents: Glucose is a reducing sugar, meaning it has the ability to donate electrons and undergo oxidation reactions. It can react with oxidizing agents such as Benedict’s reagent or Tollens’ reagent, forming a colored precipitate or a silver mirror, respectively. These reactions are commonly used in laboratory tests to detect the presence of glucose.
- Fermentation: Glucose can undergo fermentation in the presence of yeast or certain microorganisms. Through the process of anaerobic respiration, glucose is converted into alcohol (ethanol) and carbon dioxide. This process is utilized in the production of alcoholic beverages and in the baking industry to cause dough to rise.
- Hydrolysis: Glucose can undergo hydrolysis, where it reacts with water molecules, breaking the glycosidic bonds between the glucose units. Enzymes, such as amylase, can catalyze the hydrolysis of glucose polymers like starch or glycogen, converting them into individual glucose molecules.
- Reaction with Acids: Glucose can react with acids, resulting in the formation of various products. For example, when glucose reacts with concentrated sulfuric acid, it undergoes dehydration to form furfural, a derivative used in the production of chemicals. Glucose can also undergo esterification reactions with carboxylic acids to form glucose esters.
- Polymerization: Glucose can undergo polymerization reactions to form larger carbohydrates. Through condensation reactions, glucose molecules can join together, forming glycosidic bonds and creating disaccharides (such as maltose or sucrose) or polysaccharides (such as cellulose or starch). These polymers serve as energy storage molecules or structural components in organisms.
- Maillard Reaction: Glucose can participate in the Maillard reaction, a chemical reaction between reducing sugars and amino acids or proteins. This reaction occurs during cooking and leads to the browning and development of flavors in foods. The Maillard reaction is responsible for the aroma, color, and taste of various cooked or processed foods.
Uses of Glucose
- Energy Source: Glucose serves as a primary source of energy for living organisms, including humans. It is metabolized through cellular respiration to produce ATP (adenosine triphosphate), the energy currency of cells.
- Food and Beverage Industry: Glucose is widely used in the food and beverage industry. It is commonly added as a sweetening agent and flavor enhancer in a variety of products, including candies, confectioneries, baked goods, beverages, and desserts.
- Pharmaceutical Applications: Glucose has several pharmaceutical applications. It is used in the formulation of oral solutions, syrups, and suspensions as a sweetening agent and vehicle for drug delivery.
- Medical Diagnostics: Glucose is widely used in medical diagnostics. Blood glucose measurements are commonly performed to monitor and diagnose conditions such as diabetes mellitus. Glucose is also used in diagnostic kits and test strips for glucose monitoring devices.
- Biotechnology and Fermentation: Glucose is utilized in biotechnological processes and fermentation. It serves as a substrate for the growth and production of microorganisms, including the production of enzymes, antibiotics, and biofuels. Glucose is a crucial component in the fermentation of alcoholic beverages, as it is converted into alcohol and carbon dioxide by yeast.
- Chemical and Industrial Applications: Glucose is utilized in various chemical and industrial applications. It is a precursor for the production of chemicals, such as organic acids, solvents, and derivatives like furfural. Glucose polymers, such as cellulose and starch, are used in industries like papermaking, textiles, adhesives, and bioplastics.
- Research and Laboratory Use: Glucose is extensively used in research and laboratory settings. It serves as a substrate for culturing microorganisms and cells in microbiology and cell culture studies. Glucose is also utilized in biochemical and enzymatic assays, as well as in the synthesis of other compounds for research purposes.
Conclusion
In conclusion, glucose is a critical molecule that serves as the primary energy source for cells and plays a fundamental role in metabolism. It is involved in various physiological processes and is necessary for the proper functioning of the body. Maintaining balanced glucose levels is essential for overall health and well-being.
Solved Examples on Glucose
Example 1: Calculation of glucose concentration:
Given the mass of glucose and the volume of the solution, we can calculate the concentration of glucose in the solution.
Eg: If you have 10 grams of glucose dissolved in 500 mL of water, what is the concentration of glucose in the solution?
Solution: Concentration = (mass of solute / volume of solution) * 100 Concentration = (10 g / 500 mL) * 100 = 2%
Example 2: Calculation of glucose intake:
To determine the amount of glucose consumed based on the grams of carbohydrates in a food item, we can calculate the glucose intake.
Eg: If a food item contains 25 grams of carbohydrates, how much glucose does it provide?
Solution:
Glucose intake = grams of carbohydrates
Glucose intake = 25 grams
Frequently Asked Questions on Glucose
Why is glucose called sugar?
Glucose is called sugar because it falls into the category of carbohydrates known as simple sugars. It is a monosaccharide, which means it consists of a single sugar unit. Glucose is one of the most important and abundant sugars found in nature and serves as a primary source of energy for living organisms.
What's in glucose?
Glucose is a molecule composed of carbon, hydrogen, and oxygen atoms. Its chemical formula is C6H12O6, indicating that it consists of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
What is a glucose normal level?
A normal blood glucose level, measured in milligrams per deciliter (mg/dL) or millimoles per liter (mmol/L), can vary slightly depending on the specific guidelines used by different organizations. However, a commonly accepted range for fasting blood glucose (glucose level after an overnight fast) is around 70-99 mg/dL (3.9-5.5 mmol/L). Postprandial (after eating) blood glucose levels can rise temporarily, but they generally should not exceed 140 mg/dL (7.8 mmol/L) two hours after a meal.
What is glucose to sugar?
Glucose is a type of sugar. Specifically, it is a simple sugar or monosaccharide. Other types of sugars include fructose (found in fruits and honey), sucrose (table sugar composed of glucose and fructose), lactose (milk sugar composed of glucose and galactose), and maltose (found in grains and malt products, composed of two glucose molecules).
What is another name for glucose?
Glucose is sometimes referred to as dextrose. Dextrose is the naturally occurring form of glucose that is found in various foods, including fruits, vegetables, and honey. The terms glucose and dextrose are often used interchangeably.
What are the 4 types of sugar?
The four main types of sugar are: Glucose: Also known as blood sugar or dextrose, it is the primary source of energy for cellular processes.
b) Fructose: This sugar is found in fruits and some vegetables and is sweeter than glucose. It is commonly used as a sweetener in processed foods and beverages.
c) Sucrose: Also known as table sugar, it is composed of glucose and fructose. It is obtained from sugar cane or sugar beets and is widely used in cooking and food preparation.
d) Lactose: This sugar is found in milk and dairy products. It consists of glucose and galactose and is the primary carbohydrate in milk.
Where is glucose found?
Plants: Glucose is produced through photosynthesis in plants and is stored as starch or converted to sucrose for transport. Food sources: Glucose is present in many foods that contain carbohydrates, such as fruits, vegetables, grains, and sweeteners like honey and corn syrup. Human and animal bodies: Glucose is a crucial component of the bloodstream and serves as the primary energy source for cells. It is produced through the breakdown of carbohydrates during digestion or synthesized through processes like gluconeogenesis in the liver.








