Table of Contents
Introduction
- Each element has a unique symbol, typically consisting of one or two letters derived from its name.
- For example, the symbol for Hydrogen is H, for Carbon is C, and for Oxygen is O.
- The symbols are used to represent the elements in chemical formulas and equations.
- The periodic table is organized based on the atomic number, which represents the number of protons in an atom’s nucleus.
- Elements are categorized into different groups and periods based on their properties and electron configurations.
- Some symbols are derived from the element’s English name, while others are derived from their Latin or Greek names.
- Symbols can consist of one uppercase letter, such as H for Hydrogen, or an uppercase letter followed by a lowercase letter, such as Na for Sodium.
- Elements from similar groups often have similar chemical properties, while elements in the same period have the same number of electron shells.
- The periodic table includes various types of elements, including metals, nonmetals, and metalloids.
- Symbols are used universally in scientific literature and communication to represent the elements, providing a concise way to refer to each element.
Remember, the periodic table consists of 118 elements, each with its own unique symbol. The symbols play a crucial role in understanding and studying the properties and interactions of these elements.
Definition of Symbol of an Element
The symbol of an element is a shorthand representation used to identify and refer to a specific chemical element. It is typically a one- or two-letter abbreviation derived from the element’s name, often in English or Latin. The symbols are standardized and universally recognized, allowing scientists and researchers to communicate and refer to elements in a concise and unambiguous manner.
Also Check
Periodic Table
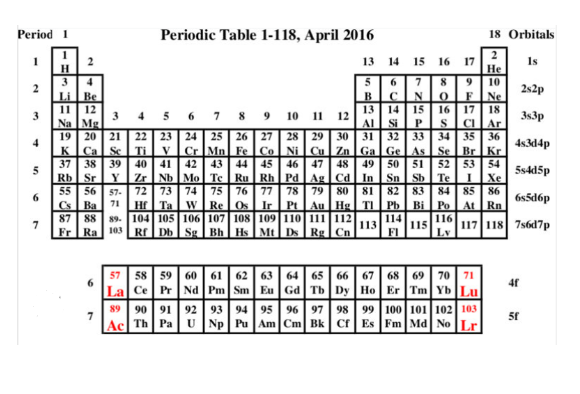
Rules or Conventions followed to denote the Element using Symbol
To denote elements using symbols, there are some rules and conventions followed:
- Symbols are typically derived from the name of the element:
- The first letter of the element’s English name is used as the symbol for most elements. For example, “H” for Hydrogen, “O” for Oxygen.
- If two elements start with the same letter, the second letter of their name is used as well. For example, “He” for Helium, “Hg” for Mercury.
- Symbols are case-sensitive:
- Most symbols are written using an uppercase letter for the first letter and lowercase letters for subsequent letters. For example, “Na” for Sodium.
- However, some symbols are derived from proper nouns or specific names and are capitalized entirely. For example, “Ra” for Radium.
- Symbols may differ from the element’s name in English:
- Symbols are sometimes derived from the element’s Latin or Greek name. For example, “Fe” for Iron (derived from the Latin word “ferrum”).
- Symbols may also represent the element’s name in a different language. For example, “W” for Tungsten (derived from the German word “Wolfram”).
- Symbols for elements with similar names or properties may have related symbols:
- Elements within the same group or with similar chemical properties may have symbols derived from a common root. For example, all noble gases (Group 18) end with the “-on” suffix: “He” for Helium, “Ne” for Neon, “Ar” for Argon, etc.
- The symbols are internationally standardized:
- The symbols used for elements follow international standards and are recognized worldwide.
- They provide a universal means of identifying and representing elements in scientific literature, research, and communication.
Conclusion
In conclusion, elements are represented by symbols, which are shorthand abbreviations derived from their names. These symbols are used to identify and refer to specific chemical elements in a concise and standardized manner. They play a crucial role in chemical formulas, equations, and the periodic table.
The symbols follow certain rules and conventions. They may consist of one or two letters, usually derived from the element’s English, Latin, or Greek name. Symbols can be uppercase, lowercase, or a combination of both, and they are case-sensitive.
The symbols and atomic numbers of elements provide important information about their identities and characteristics. By understanding and recognizing the symbols, scientists and researchers can communicate effectively and study the properties and interactions of various elements.
Solved Examples on 118 Elements with their Symbols
Example 1: If an element has an atomic number of 17, what is its symbol?
Solution: The element with atomic number 17 is Chlorine (Cl).
Example 2: If an element has a symbol of Au, what is its atomic number?
Solution: The element with the symbol Au is Gold. Its atomic number is 79.
Example 3: An element has an atomic number of 26. What is its symbol?
Solution: The element with atomic number 26 is Iron. Its symbol is Fe.
Example 4: If an element has a symbol of Mg, what is its atomic number?
Solution: The element with the symbol Mg is Magnesium. Its atomic number is 12.
Example 5: An element has an atomic number of 8. What is its symbol?
Solution: The element with atomic number 8 is Oxygen. Its symbol is O.
FAQs on 118 Elements with their Symbols
What is the IUPAC symbol of atomic number 119?
According to the rule of IUPAC element having atomic number, more than 100 can be named by using numbers of its atomic number. Here 1 is written as Un and 9 is written as en and the name end with ium. Thus the name of the given compound will be Ununennium and symbol will be Uue.
Is there a 120th element?
Unbinilium, also known as eka-radium or simply element 120, is the hypothetical chemical element in the periodic table with symbol Ubn and atomic number 120.
What are the 1 to 60 elements?
The 1 to 60 elements include Hydrogen (H) to Neodymium (Nd) in the periodic table.
What is the symbol of 1 to 30 elements?
Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron (B) Carbon (C) Nitrogen (N) Oxygen (O) Fluorine (F) Neon (Ne) Sodium (Na) Magnesium (Mg) Aluminum (Al) Silicon (Si) Phosphorus (P) Sulfur (S) Chlorine (Cl) Argon (Ar) Potassium (K) Calcium (Ca) Scandium (Sc) Titanium (Ti) Vanadium (V) Chromium (Cr) Manganese (Mn) Iron (Fe) Cobalt (Co) Nickel (Ni) Copper (Cu) Zinc (Zn) Gallium (Ga)
How to remember all 118 elements?
Remembering all 118 elements can be challenging, but mnemonic techniques can help. Some common methods include creating acronyms, associating elements with memorable images or stories, or using visual aids like flashcards or periodic table charts. Breaking the elements into smaller groups or categories and learning them systematically can also aid in memorization.
What is 118 table of elements?
The term 118 table of elements refers to the periodic table, which is a tabular arrangement of all the chemical elements, organized based on their atomic number, electron configurations, and recurring chemical properties.
Has element 119 been made?
It is the lightest element that has not yet been synthesized.
What is the use of 118 element?
The 118th element is Oganesson (Og), which is a synthetic element and the heaviest element currently known. Oganesson is highly unstable and has a very short half-life, making it difficult to study and utilize in practical applications. As of now, there are no known uses for Oganesson in everyday life or industrial applications. Its discovery and study primarily contribute to our understanding of the periodic table and the properties of heavy elements.








