Table of Contents
Rutherford’s Model of the Atom
Introduction:
Rutherford’s model of the atom: With his renowned gold-foil experiment, Rutherford disproved Thomson’s hypothesis in 1911, demonstrating that the atom has a tiny, heavy nucleus. Rutherford had discovered five years before that alpha particle beamed in via a hole onto a photographic plate produced a sharp-edged image, whereas alpha particles beamed through a 20 micrometre (or roughly 0.002 cm) thick sheet of mica produced an impression with hazy edges. The young physicists used gold foil to beam alpha particles, which they detected as flashes of light or scintillations on a screen. The gold foil has a thickness of only 0.00004 cm. The majority of the alpha particles simply passed through the foil, but some were redirected and hit a point on a screen that was set out to the side.
Despite the fact that early atomic models were erroneous and unable to adequately explain the structure of the atom and experimental results. However, it served as the foundation for quantum mechanics and aided in its future growth. A model of an atom was produced during the experiment to demonstrate that a large amount of space in an atom is vacant. A considerable number of the -particles went right through the gold layer without being deflected. As a result, an atom’s interior must be largely empty of substance. The positive charge in an atom is still not equally distributed throughout the atom but rather concentrated in a small area — the nucleus. The gold sheet was able to deflect a tiny number of -particles when assaulted with them. They were deflected using tiny angles and deflections.
A brief outline
The Rutherford atomic model was viewed with suspicion by many physicists as it was hard to reconcile with the chemical behaviour of atoms. According to the idea, the nucleus’ charge was the most essential property of the atom, dictating its structure. Mendeleyev’s periodic table, on the other hand, was ordered as per the atomic masses of the elements, meaning that atoms’ structure and chemical behaviour were determined by their mass.
Experiment with the Rutherford Atomic Model
In Rutherford’s experiment, high-energy streams of -particles were battered on a thin gold foil with a thickness of 100 nm. The -particle streams were ejected from a radioactive source. He carried out the experiment to investigate the deviation in the track of -particles after they collided with a thin sheet of gold. He surrounded the gold foil with a screen constructed of zinc sulphide to analyze the deflection. Rutherford’s observations were in direct opposition to J.J. Thomson’s plum pudding model.
Important concepts
Rutherford Model Experiment Observations
- Rutherford came to the conclusion that based on the observations obtained throughout the experiment
- A high fraction of -particles travelled through gold sheets without being deflected, indicating that the majority of space in an atom is unoccupied. As a result, the vast majority of an atom must be empty.
- The positive charge inside an atom is not evenly distributed and is concentrated in a limited area – The gold sheet deflected a small number of -particles when they were blasted. They were deflected at very small angles and deflected minutely. In a nutshell, he summarized the findings.
- Only a few -particles had deflected back or deflected at enormous angles. Furthermore, just a few particles deflected at 180ͦ
- As a conclusion, he came to the realization that the positively charged particles only covered a tiny fraction of an atom’s overall volume.
Rutherford’s atomic model theorizes based on observations and findings
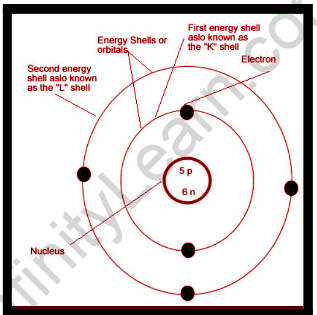
- Positively charged particles make up an atom. The majority of an atom’s mass was contained in a very small area. The nucleus of an atom was named after this part of the atom. The very tiny and intense nucleus of an atom is made up of neutrons and protons, as was discovered later.
- The nucleus of an atom is surrounded by electrons, which are negatively charged particles. The electrons travel in a fixed circular path around the nucleus at a very high speed. “Orbits” were given to these fixed circular pathways.
- Although electrons are negatively charged and the tightly concentrated nucleus is positively charged, an atom has no net charge or is electrically neutral. The nucleus and electrons are held together by a strong electrical attraction.
- The nucleus of an atom is extremely small when compared to the whole size of the atom.
Rutherford Atomic Model Limitations
- The persistence of an atom was not explained by Rutherford’s idea. According to Rutherford’s assumption, electrons in a fixed orbit rotate at a very high speed around the nucleus of an atom.
- Maxwell, from the other perspective, demonstrated that accelerated charged particles release electromagnetic radiations. Electrons spinning all around the nucleus will emit electromagnetic radiation as a result.
- The electromagnetic radiation will gain energy from the electrical action, causing the orbits to shorten over time. In the nucleus of an atom, the orbits will eventually shorten and collapse. As per the estimates, Rutherford’s model will collapse in 10-8 seconds if Maxwell’s explanation is followed. As a result, the Rutherford atomic model did not follow Maxwell’s theory and was unable to explain the stability of an atom.
- Rutherford’s idea was flawed because it omitted information on the electron arrangement in the orbit. One of the key flaws in the Rutherford atomic model was this.
Significance of Rutherford’s model of the atom
The Rutherford model NEET conversations are depended upon to uncover and offer responses to the most often introduced demands on the test. With the assistance of notes from proficient experts in the field, which is given on the Infinity Learn online stage, these can be clarified in fundamental terms. Expecting that understudies manage heightened energy for the subjects commonly through the informative program, different decision requests are obvious to rehearse.
Prepared experts and talented instructors in the subject give responses. The responses are as per CBSE and NCERT rules for the NEET test, helping understudies in accomplishing higher outcomes. The courses are sensibly evaluated, and there are several free courses open for new understudies to test. On an equivalent stage, there is a substitute assurance of courses going from kindergarten to twelfth grade, as well as unequivocal exercises for awful tests like NEET.
FAQs (Frequently asked questions)
Question 1: Include Rutherford’s conclusions from his -ray scattering experiment.
Ans: From his -ray scattering experiment, Rutherford came to the following conclusion.
- particles flowed only through gold foil with no deflection, confirming the atom’s empty space.
- Few particles show deflection, indicating that the positive charge of the atom takes up relatively little space.
- Deflection in the field All of the positive charge and weight of the gold atom was concentrated in a relatively small volume within the atom, as indicated by the minuscule proportion of -particles.
In his –ray scattering experiment, why did Rutherford use gold foil?
Rutherford envisioned a metal sheet that could be as thin as the scattering experiment required. Gold is by far the most malleable of all known metals. It's simple to cut into very thin sheets. As a result, Rutherford picked gold foil for his alpha-ray scattering experiment.
Question 3: Why was it important to keep the Rutherford experiment in a vacuum?
Ans: In the Rutherford experiment, a positively charged helium ion travelling with kinetic energy collides with another positively charged helium ion. The influence on subatomic particles is determined by the helium ion’s positive charge and kinetic energy.
The medium will give resistance to the flow of the helium ions in the air or other gaseous mediums, reducing their kinetic energy. Furthermore, due to their positive character, helium ions may collide with air particles, ionise them, and fail to reach the gold foil. In order for the helium ion to hit the gold foil, the chamber must maintain a vacuum.








