Table of Contents
Introduction
John Dalton initially proposed the theory of chemical combination in 1803. It is based on the following premises:
(1) Elements are made up of microscopic indivisible particles (atoms).
(2) Atoms of the same element are all identical, whereas atoms of different elements are of distinct sorts.
(3) It is impossible to produce or destroy an atom.
(4) When atoms of different elements combine in simple ratios to generate ‘compound atoms,’ ‘compound elements’ (i.e. compounds) are formed (i.e. molecules).
Dalton also offered symbols for several elements’ atoms (later replaced by the present notation using letters). Dalton’s atomic theory was by far his most influential work in chemistry. Attempts to trace Dalton’s development of this theory have failed; even Dalton’s own recollections on the subject are incomplete. He based his partial pressure theory on the idea that only like atoms in a gas mixture repel one another, whereas unlike atoms appear to react indifferently to one another. This model explained why each gas in a mixture behaved independently. Although this view was later proven to be incorrect, it served a useful purpose in allowing him to abandon the notion, held by many previous atomists from the Greek philosopher Democritus to the 18th-century mathematician and astronomer Ruggero Giuseppe Boscovich, that atoms of all kinds of matter are the same.
Dalton asserted that atoms of different elements differ in size and mass, and this assertion is central to his atomic theory. His claim that each element had its own type of atom seemed counterintuitive to those who believed that having so many different fundamental particles would destroy nature’s simplicity, but Dalton dismissed their concerns as fanciful. Instead, he concentrated on determining the relative masses of various types of atoms, a process he claimed could only be accomplished by taking into account the number of atoms of each element present in various chemical compounds. Dalton had been teaching chemistry for several years, but he had not yet conducted actual research in this field.
Overview
Dalton’s atomic theory was the first comprehensive attempt to characterize all matter in terms of atoms and their properties. Dalton’s theory was founded on the laws of mass conservation and constant composition. The first part of his theory states that all matter is made up of indivisible atoms. According to the second part of the theory, all atoms of a given element have the same mass and properties. Compounds are made up of two or more different types of atoms, according to the third party. According to the fourth part of the theory, a chemical reaction is a reorganization of atoms. Because of the discovery of subatomic particles and isotopes, parts of the theory had to be revised.
The law of mass conservation and the law of constant composition were the foundations of Dalton’s hypothesis. According to the law of conservation of mass, the matter is not created or destroyed in a closed system. That is, if we have a chemical reaction, the amount of each element in the starting materials and the products must be the same.
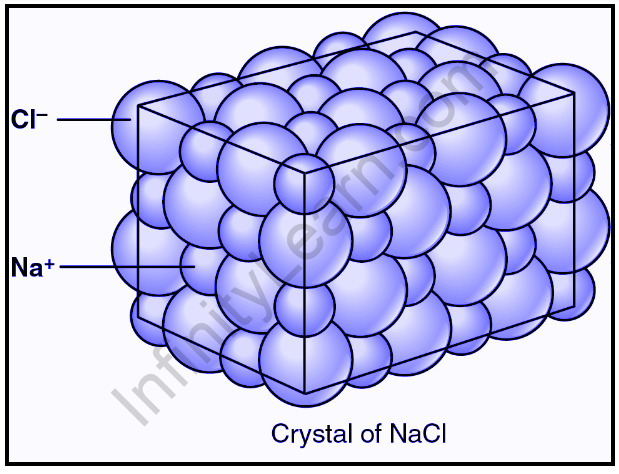
According to the law of constant composition, a pure compound will always contain the same proportion of the same elements. We could make more table salt with the same composition by combining sodium metal and chlorine gas, which I would not recommend doing at home.
Dalton’s atomic theory
Dalton proposed that the concept of atoms could explain the laws of conservation of mass and definite proportions. He proposed that all matter is composed of tiny indivisible particles known as atoms, which he envisioned as “solid, massy, hard, impenetrable, movable particle(s).” It is important to note Dalton lacked the tools to observe or experiment on individual atoms, therefore he had no way of knowing if they had any interior structure. We could imagine Dalton’s atom as a piece in a molecular modeling kit, with different elements represented by spheres of varying sizes and colors. While this model is useful for some applications, we now know that atoms are not solid spheres. Dalton proposed that every atom of a given element, such as gold, is identical to every other atom of that element. He also observed that the atoms of one element are distinct from the atoms of all other elements. Today, we know that this is mostly true. A sodium atom is not the same as a carbon atom. Despite the fact that several elements have comparable boiling points, melting points, and electronegativities, no two elements have the exact same combination of properties.
Dalton proposed in the third part of his atomic theory that compounds are combinations of two or more different types of atoms. Table salt is an example of such a compound. Table salt is made up of two distinct elements, each with its own set of physical and chemical properties. The first element is sodium, which is a highly reactive metal. The second substance, chlorine, is a poisonous gas. The atoms combine in a 1:1 ratio to form white crystals of NaCl when they react. Dalton proposed in the fourth and final part of his atomic theory that chemical reactions do not destroy or create atoms. The atoms were simply rearranged. To return to our salt example, when sodium combines with chlorine to form salt, both the sodium and chlorine atoms remain. They simply rearrange themselves to form a new compound.
Dalton’s atomic model
Atoms are tiny, indivisible particles that make up all matter. All atoms of a given element have the same mass, size, and other properties. Atoms of different elements, on the other hand, have different properties and differ in mass and size. Atoms can neither create nor destroy themselves. In addition, atoms cannot be broken down into smaller particles. Compounds are formed when atoms of different elements combine in fixed whole-number ratios. In chemical reactions, atoms can be rearranged, combined, or separated.
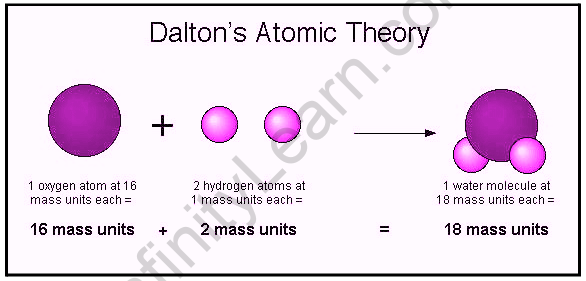
In 1808 John Dalton published his first general account of chemical atomic theory, which has since become a cornerstone of modern chemistry. The theory arose from his earlier research into the properties of atmospheric gases.
Dalton’s theory
Atoms are the building blocks of all matter. Atoms are indestructible and indivisible. The mass and properties of all atoms of a given element are the same. Compounds are created by combining two or more different types of atoms. A chemical reaction is an atom rearrangement. Of course, modern atomic theory is more complicated than Dalton’s theory, but the essence of Dalton’s theory remains valid. Atoms can be destroyed by nuclear reactions but not by chemical reactions, we now know. Also, different types of atoms (different in mass) within an element are known as “isotopes,” but isotopes of the same element have the same chemical properties. Dalton’s theory quickly explained many previously unexplained chemical phenomena. Dalton’s theory quickly established itself as the theoretical foundation of chemistry.
Crack NEET with Result-Oriented Learning Program from Infinity Learn
FAQs
What role does Dalton's atomic theory play in explaining the law of conservation of mass?
Dalton's theory suggests that the net mass of the participating species in a chemical reaction is conserved because it states that atoms cannot be created or destroyed. As a result, this postulate explains the law of conservation of mass.
Dalton's atomic theory distinguishes between elements and compounds in what way?
According to this theory, elements combine in fixed, whole-number ratios to form compounds. As a result, it implies that compounds are made up of molecules containing two or more atoms of different elements.
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Ultimate Learning App – Infinity Learn.








