Table of Contents
Silicon Tetrachloride is a great water-soluble crystalline Silicon source for chloride-compatible applications. When chloride compounds are fused or dissolved in water, they can conduct electricity. Electrolysis can decompose chloride materials into chlorine gas and metal. They are created through various chlorination processes in which at least one chlorine anion (Cl–) is covalently bonded to the relevant metal or cation. It is possible to create ultra-pure formulations and proprietary formulations. In metabolic systems, the chloride ion regulates fluid equilibrium and pH levels. They can combine to form both inorganic and organic compounds. Most volumes of silicon chloride are readily available. Forms such as ultra-high purity, high purity, submicron, and nanopowder may be considered. Silicon Tetrachloride is a great water-soluble crystalline Silicon source for chloride-compatible applications. Whenever chloride compounds are fused or dissolved in water, they can conduct electricity.
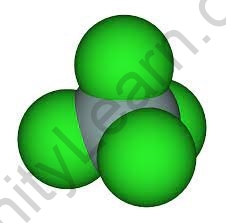
Overview
Silicon tetrachloride and chlorosilans have been used as by-products in a variety of industries, including electronics, telecommunications, and metallurgy. These materials can also be used in advanced ceramics and the chemical and automotive industries, primarily in the production of optical fibres, cutting tools, internal combustion engine components, high-speed ball bearings, mechanical seals, photovoltaic cells, integrated circuits, transistors, thyristors, and chips. Silicon tetrachloride is toxic and corrosive, and it reacts violently with water. At room temperature, silicon tetrachloride is a colourless liquid that fumes in moist air. The molecules’ only attraction is van der Waals dispersion forces. Because there are no ions or mobile electrons, it does not conduct electricity.
Based on its physical-chemical and toxicological properties, silicon tetrachloride is not intended for consumer use. Worker risk and exposure are extremely unlikely because this substance must be manufactured and handled in industrial settings under strictly controlled conditions. Surface water and soil should not be exposed to silicon tetrachloride. If the risk recommendations listed under RISK MANAGEMENT RECOMMENDATIONS are followed, the substance can be handled safely.
Silicon Tetrachloride
Silicon tetrachloride is a colourless, non-flammable, volatile liquid with a suffocating, pungent odour. It emits fumes into the atmosphere and is corrosive to metals and tissues when exposed to moisture. In Argonne National Laboratory experiments, where it was mixed with water and stirred at room temperature, about 35% of the theoretical yield of HCl evolved as a gas in the first minute. It also reacts quickly with alcohols, primary and secondary amines, ammonia, and other compounds with active hydrogen atoms. Thermal decomposition or combustion can result in dense white clouds of silicon oxide particles and hydrogen chloride.
Silicon tetrachloride is a by-product of the manufacturing of polysilicon, the key component of sunlight-capturing wafers in solar energy panels, and at least four tonnes of silicon tetrachloride liquid waste are generated for every tonne of polysilicon produced. Pollution from silicon tetrachloride has been reported in China, which has been linked to an increase in demand for photovoltaic cells fueled by subsidy programmes.
The substance is acutely toxic by oral and inhalation exposure due to its corrosivity. Corrosive effects can also occur after repeated exposure, particularly in the respiratory tract. It may cause severe skin burns and eye damage. Silicon tetrachloride has not been shown to be mutagenic or genotoxic. There is no evidence that silicon tetrachloride is a reproductive or developmental toxin, nor is it carcinogenic.
Silicon tetrachloride degrades to silicic acid and hydrogen chloride when hydrolyzed. Silicon tetrachloride is considered to be low toxicity to aquatic organisms based on available data for hydrolysis products. As an inorganic substance, biodegradation tests are inapplicable.
There are no downstream uses that would result in consumer exposure, and all intended uses are industrial only. Silicon tetrachloride is highly reactive, and no unreacted starting material remains in the end products.
Because of the corrosive and reactive nature of the substance, all aspects of Silicon tetrachloride handling, including on-site storage and transfer, must be subject to highly controlled conditions to reduce the risk of worker exposure. Because silicon tetrachloride is highly reactive and unstable, there is no environmental exposure to humans.
Because silicon is larger, the water molecule has more room to attack; the transition is less cluttered. Silicon also has an advantage in that there are empty third orbitals available to accept a lone pair from the water molecule.
As a result, the oxygen atom can bond to silicon before a silicon-chlorine bond breaks, making the entire process less energetically demanding. Silicon tetrachloride reacts violently with water, resulting in white solid silicon dioxide and HCl gas.
Silicon Tetrachloride Formula
Silicon tetrachloride is an inorganic compound with the formula SiCl4, and it is a colourless volatile liquid that emits fumes into the atmosphere. It can be used to make high-purity silicon and silica for commercial use.
Silicon Tetrachloride Uses
Silicon tetrachloride is a raw material/intermediate used in the manufacture of silicon, silica, and other silicon-based substances. Silicon tetrachloride is the raw material used to make pure silicon, which is then used to make semiconductors and optical fibers. The substance is manufactured and handled in closed systems in industrial settings.
FAQs
What is silicon tetrachloride used for?
Silicon tetrachloride is often used as a raw material/intermediate in the production of silicon, silica, and other silicon-based substances. Silicon tetrachloride is the starting material for the production of pure silicon, which is used to make semiconductors and optical fibers.
What happens when silicon tetrachloride reacts with water?
When silicon tetrachloride reacts with water, it produces white solid silicon dioxide and HCl gas.
Is silicon tetrachloride soluble in water?
Silicon Tetrachloride is a great water-soluble crystalline Silicon source for chloride-compatible applications. When chloride compounds are fused or dissolved in water, they can conduct electricity.
How is silicon tetrachloride formed?
Silicon tetrachloride is made by chlorinating silicon compounds such as ferrosilicon, silicon carbide, or silicon dioxide/carbon mixtures.
Why is silicon tetrachloride a liquid?
At room temperature, silicon tetrachloride is a colourless liquid that fumes in moist air. The molecules' only attraction is van der Waals dispersion forces. Because there are no ions or mobile electrons, it does not conduct electricity.






