Table of Contents
In a single covalent bond, both atoms contribute one valence electron to form a shared pair. A valence electron is an electron linked with an atom in the outer shell that can participate in the formation of a chemical bond if the outer shell is not closed. The presence of valence electrons can influence an element’s chemical properties, such as its valence—whether it can bond with other elements and, if so, how easily and how many times. In this sense, an element’s reactivity is significantly influenced by its electrical arrangement. A valence electron can only exist in the outermost electron shell of a main-group element; a valence electron can also exist in an inner shell of a transition metal.
A chemically inert atom has a closed shell of valence electrons (corresponding to a noble gas configuration). Atoms with one or two extra valence electrons than a closed shell are highly reactive because the energy required to remove the extra valence electrons to form a positive ion is relatively low. An atom with one or two electrons fewer than a closed shell is reactive because of its proclivity to either gain the missing valence electrons and form a negative ion or to build a covalent connection by sharing valence electrons. A valence electron, like a core electron, can absorb or release energy in the form of a photon. An increase in energy can cause an electron to move (jump) to an outer shell, a process known as atomic excitation. Alternatively, the electron can break free from the shell of its associated atom, resulting in the formation of a positive ion. When an electron loses energy (and thus emits a photon), it can move to an inner shell that is not completely occupied.
Overview
Valence electrons are electrons found in an atom’s outermost shell because the electrons in the outermost shells of two atoms are the first to come into contact with each other and are the ones that determine how an atom reacts in a chemical reaction when two atoms interact.
The s and p electrons in the outermost shell are valence electrons. The electrons in the inner shell are known as core electrons. When we study and observe an element’s atom, we come across tiny subatomic particles known as valence electrons. The tracking of valence electrons and the prediction of bond types are both aided by Lewis structures. Valence electrons are all negatively charged particles that are arranged in various orbitals or shells. Furthermore, these electrons are in charge of atom interaction and the formation of chemical bonds. Not all electrons, however, are associated with the atom. Only the electrons in the outermost shell are capable of participating in the formation of a chemical bond or molecule. These electrons are known as valence electrons.
The number of electrons that an atom must lose or gain in order to achieve the nearest noble gas or inert gas electronic configuration is referred to as valence. “Valence electrons are electrons in the outer shells that are not filled.” Valence electrons are involved in the majority of chemical reactions because they have more energy than electrons in inner orbits. Meanwhile, the number of valence electrons present aids in determining the chemical properties of a specific element, such as its valence or valency and the formation of bonds with other elements. It also tells us how easily the atoms can form bonds, how many unpaired electrons there are, and how many atoms can participate. Valence electrons are involved in the majority of chemical processes because they have more energy than electrons in inner orbits. They help us determine an element’s chemical properties, such as its valency or how it forms bonds with other elements. It also tells us how easily atoms can form bonds, how many unpaired electrons exist, and how many atoms can take part.
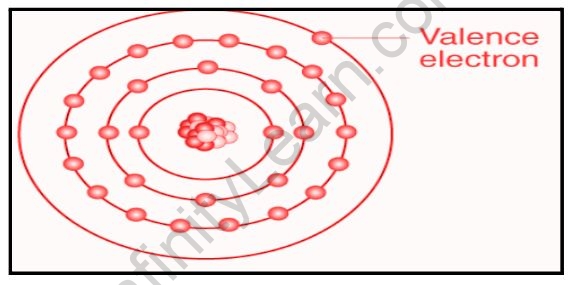
Valence electrons are any of the fundamental negatively charged particles in the outermost region of atoms that participate in the formation of chemical bonds. Regardless of the type of chemical connection (ionic, covalent, or metallic) between atoms, changes in atomic structure are confined to the outermost, or valence, electrons. They are less strongly attracted to the positive atomic nucleus than the inner electrons and can thus be shared or transferred with nearby atoms during the bonding process. In metals and semiconductors, valence electrons are also involved in the conduction of electric current.
Valency
The valency of an element is the number of electrons lost, gained, or shared by an atom of the element during a chemical reaction in order to complete its octet. An element with atomic number 14 will have a valency of 4 due to electron sharing because it has 4 electrons in its outermost shell (2,8,4) electronic configuration.
Some examples of elements are given below, along with their valency. Let’s look at some examples to better understand the valency.
- Copper is a transitional element. The majority of the transition elements have variable valences. Copper is classified into two valences: 1 and 2. When copper has valency 1 or Cu(I), it is known as Cuprous, and when it has valency 2 or Cu(II), it is known as Cupric.
- Nitrogen has an atomic number of 7. It has an electronic configuration of 2 and 5. As a result, it is obvious that nitrogen has 5 electrons in its outermost shell. Nitrogen requires three more electrons to complete its octet. It will achieve stability after completing its octet. As a result, the valency of nitrogen is 3.
- Sodium has an atomic number of 11. It has a 2,8,1 electronic configuration. As a result, it is clear that sodium requires one electron to lose in order to achieve stability by completing its octet. As a result, it has a valency of 1 as a result.
- Fluorine has an atomic number of 9. It has a 2,7 electronic configuration. It requires one electron to complete its octet and achieve stability. It has a valency of 1 as a result.
- Lithium has an atomic number of three. It has a two-to-one electronic configuration. As a result, it must lose one electron in order to achieve stability and an electronic configuration similar to that of the noble gas Helium. As a result, it has a valency of 1 as a result of this.
Valency of chlorine
An atom’s valency relates to its capacity to interact with other atoms. The valency of an element expresses the number of bonds that an atom can form as part of a compound. The amount of hydrogen atoms that can join with or replace one of an element’s atoms is known as its valence (directly or indirectly). When the number of electrons in an atom’s outer shell is four or less, its valence is equal to that number. The outer shell valence is then equal to eight minus the number of electrons.
Chlorine has an atomic number of 17.
Chlorine’s electronic configuration is[Ne]3s23p5, which can also be written as 2,8,7. This demonstrates that chlorine has seven valence electrons in its outermost shell. If the number of valence electrons is greater than four, the valency can be calculated as follows:
valency = electron valence -8
As a result, the valency of chlorine is 7-8, which equals 1.
As a result, chlorine has a valency of 1.
Valency table of elements
The valence or valency of an element is the measure of its capacity to combine with other atoms when forming chemical compounds of the molecule. The following is a list of the valencies of elements with symbols.
| Element | Atomic number | Valency |
| Valency of Hydrogen | 1 | 1 |
| Valency of Helium | 2 | 0 |
| Valency of Lithium | 3 | 1 |
| Valency of Beryllium | 4 | 2 |
| Valency of Boron | 5 | 3 |
| Valency of Carbon | 6 | 4 |
| Valency of Nitrogen | 7 | 3 |
| Valency of Oxygen | 8 | 2 |
| Valency of Fluorine | 9 | 1 |
| Valency of Neon | 10 | 0 |
Also read: Important Topic of Chemistry: Valency
FAQs
Where do you look for the valence electrons?
The number of valence electrons in neutral atoms is equal to the atom's main group number. The periodic table column containing an element's main group number can be used to determine its main group number. Carbon, for example, belongs to group 4 and has four valence electrons. Oxygen is a member of group 6 and has six valence electrons.
With an example, what is a valence electron?
The total number of electrons in the last shell orbit is referred to as the valence electron. As an example: Oxygen has 6 electrons in the last orbital shell, so 6 is the valence electron.
What exactly is valency?
An element's valency is its ability to combine with other elements. Elements in the same periodic table group have the same valency. The valency of an element is proportional to the number of electrons in its outer shell.






