Table of Contents
Any of the infinite numbers of possible spatial arrangements of atoms in a molecule that result from the rotation of its constituent groups of atoms about single bonds is referred to as a confirmation. Any molecule in which a single covalent bond connects two polyatomic groups, at least one of which does not lie along the axis of the single bond, can have multiple conformations. The most basic of these molecules are hydrogen peroxide, in which the two hydroxyl groups can rotate with respect to one another around the axis of the oxygen-oxygen bond. The presence of more than one such single bond in a molecule, as in propane, simply adds to the complexity without changing the nature of the situation. All the atoms in molecules like cyanogen or butadiyne lie along the axis of the central single bond, so no distinguishable conformations exist.
In fact, each distinguishable conformation of a molecule represents a different potential energy state as a result of the operation of attractive or repulsive forces that vary with the distances between different parts of the structure. If these forces did not exist, all conformations would have the same energy, and rotation around a single bond would be completely free or unrestricted. If the forces are strong, different conformations have a large difference in energy or stability: the molecule will normally occupy a stable state (one with low energy) and will only transition to another stable state after absorbing enough energy to reach and pass through the unstable intervening conformation.
Intramolecular forces in ethane, for example, are so weak that their existence can only be inferred from subtle changes in thermodynamic properties like enthalpy and entropy. (Ethane’s three most stable conformations are indistinguishable even if internal rotation is severely restricted.) Certain more complex compounds’ molecular structures, on the other hand, impose such strong barriers to a rotation that stereoisomeric forms—differing only in conformation—can be isolated.
Conformational Isomers
In general, conformations or conformational isomers have different spatial arrangements of atoms in a molecule but the same bond connectivity and, if chiral, the same configuration. These various arrangements are produced by rapid rotations around single bonds, resulting in changes in dihedral angles between the vicinal groups involved. Because the energy barriers required to change one conformational isomer to another are usually low, interconversion of conformational isomers is possible at room temperature.
Conformational Isomerism is a type of stereoisomerism in which isomer interconversions are possible via rotations referring to single bonds. These are known as conformational isomers. In the case of single bond rotation, rotational energy acts as a barrier. To interconvert one conformer to another, it must be overcome. For Conformational Isomerism to occur, the energy barrier must be below. There are various kinds of conformational isomers. Ethane and Butane are two examples.
Conformation of Cyclohexane
Because of its non-polar structure, cyclohexane is almost free of ring strain. Chain conformation and boat conformation are two of the most important conformations that it can have. We can say that the boat conformation is less stable than the chair conformation. The boat conformation can be made more stable than usual by a slight rotation of the C-C bonds, and this is known as the skew boat conformation. Nonetheless, the chair conformation is the most stable form of cyclohexane.
A cyclohexane conformation can refer to any of the many three-dimensional shapes that a cyclohexane molecule can take without disrupting the integrity of its chemical bonds.
Internal angles of a regular hexagon shape are 120 degrees. The carbon-carbon bonds in the cyclohexane ring, on the other hand, have tetrahedral symmetry, with bond angles corresponding to 109.5 degrees.
This is why the cyclohexane ring tends to adopt several warped conformations (bringing bond angles closer to the tetrahedral angle (109.5 degrees) and reducing overall strain energy).
The boat, twist-boat, chair, and half-chair conformations are examples of common cyclohexane conformations, which are named after the shape that the cyclohexane molecule takes in them.
It should be noted that the cyclohexane molecule can switch between the conformations listed above, but only the chair and twist-boat conformations can be isolated into their pure forms.
The bond length and bond angle in these conformations vary slightly from their nominal values due to hydrogen-hydrogen interactions.
Cyclohexane chair conformations have lower energies than boat forms. The rather unstable boat forms of cyclohexane, on the other hand, undergo rapid deformation to give twist-boat forms, which are the local minima corresponding to the total energy.
Axial hydrogens are hydrogen atoms that belong to carbon-hydrogen bonds that are perpendicular to the mean plane, whereas equatorial hydrogens are hydrogen atoms that belong to carbon-hydrogen bonds that are parallel to the mean plane. These are also known as axial and equatorial bonds, respectively.
Cyclohexane is perhaps the most common ring found in natural compounds. Its prevalence, which is undoubtedly due to its stability, makes it the most important of the cycloalkanes. Because the bond angle deviation in cyclohexane molecules is greater than that in cyclopentane, it should be more strained and less reactive than cyclopentane. It is, however, less strained and more stable than cyclopentane.
To avoid the strain, cyclohexane does not exist as a planar molecule as one would expect. It exists as a puckered non-planar ring with bond angles close to tetrahedral bond angles. The boat and chair conformations are two types of puckered rings for cyclohexane.
Conformation of Ethane
Ethane is a chemical compound that is organic and it is a colourless, odourless gas at room temperature. The ethane molecule is made up of seven sigma bonds. When about six carbon-hydrogen bonds rotate, the shape of the molecule changes. When the carbon-carbon bond rotates, however, many differences can occur.
When we take the ethane ball and stick model and rotate one carbon atom while keeping another carbon atom stationary about the C-C axis. We will see that the rotations will produce an infinite number of spatial arrangements of hydrogen atoms attached to one carbon atom in relation to the hydrogen atoms attached to the other carbon atom. These various arrangements are referred to as conformational isomers or conformers.
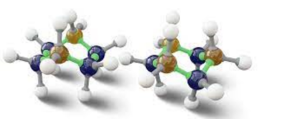
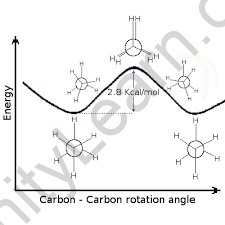
If we rotate the CH3 group clockwise at a 60-degree angle, it is possible that the hydrogen present at the front carbon is close to the hydrogen present at the back carbon. Eclipsed Conformation is what it’s called. The Eclipse Conformation is one of the highest. Another clockwise rotation at a 60-degree angle would result in the second eclipsed conformation. The solid line in the above figure represents the 6 carbon-hydrogen bond that is extended at a 120-degree angle from two carbons.
Whereas any sigma bond can have an infinite number of conformations, two specific conformers in ethane stand out and have their own names. The C-H bonds on the front and back carbons are aligned with dihedral angles of 0 degrees in the eclipsed conformation. With dihedral angles of 60 degrees, the C-H bonds on the rear carbon lie between those on the front carbon in the staggered conformation.
FAQs
What is conformation for example?
Conformation is the shape adopted by a molecule as a result of rotation around one or more single bonds. In the case of alkanes, for example, electrons are distributed around the internuclear axis of the C-C bond.
What is a conformational isomer in organic chemistry?
Conformational isomers are isomers that share the same bond connectivity sequence and can be interconverted by rotating around one or more single bonds.






