Table of Contents
Nitrobenzene is formed when benzene reacts with concentrated nitric acid at 323-333k in the presence of concentrated sulphuric acid. This is referred to as benzene nitration. The reaction nitration of benzene is accomplished by treating benzene with concentrated sulfuric acid and concentrated nitric acid at a temperature not exceeding 50C. As the temperature rises, more than one nitro group, -NO2, is more likely to be substituted onto the ring, resulting in the formation of Nitrobenzene. This reaction requires a catalyst, which is concentrated sulfuric acid. In this case, the electrophile is NO+2, also known as the nitrogenium ion or nitryl cation. This compound is formed by the reaction of nitric acid and sulfuric acid.
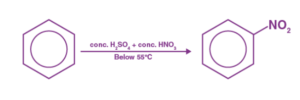
Step 1: Nitric acid accepts a proton from sulphuric acid before dissociating to form the nitronium ion.
Step 2: In the process, the nitronium ion acts as an electrophile, reacting with benzene to form the arenium ion.
Step 3: The arenium ion then loses its proton to Lewis base, resulting in the formation of nitrobenzene.
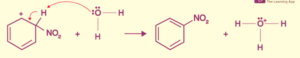
Benzene Sulfonation
When benzene and sulfuric acid are sulfonated, they undergo an electrophilic substitution reaction. The first method involves heating benzene at 40C for several hours in the presence of concentrated sulfuric acid fumes. After that, it is converted into benzenesulphonate.
The electrophile in this case is sulphur trioxide, SO3. You can make sulphur trioxide electrophile in either of two ways, depending on the type of acid used. Fumed sulfuric acid, H2S2O 7, is a SO3 solution in sulfuric acid and thus a richer source of SO3. It can also be produced by concentrated sulfuric acid containing traces of SO3. Sulfur trioxide is electrophilic due to its highly polar nature and the fact that the sulphur atom carries a very strong charge.
Sulfonation of benzene is the process of producing benzenesulfonic acid by heating benzene with fuming sulphuric acid (H2SO4 +SO3).
In nature, the reaction is reversible.
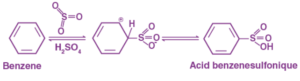
The mechanism of benzene sulfonation
Because of its higher electronegativity, the oxygen in sulphuric acid attracts an electron, resulting in the formation of an electrophile.
This attacks the benzene ring, leading to the formation of benzenesulfonic acid.
Also read: SOLUBLE PRODUCTS IN CHEMISTRY
FAQs
What exactly are alkylation and acylation?
Friedel-Crafts reactions are classified into two types: alkylation reactions and acylation reactions. The organic coupling reaction involving an electrophilic aromatic substitution that is used to attach substituents to aromatic rings is known as a Friedel-Crafts reaction. In 1877, French chemist Charles Friedel and American chemist James Crafts invented these reactions. While alkylation involves the substitution of an alkyl group for an aromatic proton, acylation involves the addition of an acyl group to an aromatic ring.
What exactly is nitrobenzene?
It is a nitro-compound that is oily, yellowish, and aromatic. It smells like almonds. When burned, it emits toxic nitrogen oxide fumes. Nitrobenzene is created through the benzene nitration reaction, in which benzene is treated with a mixture of concentrated nitric acid and concentrated sulfuric acid at a temperature of no more than 50°C. When one or more hydrogen atoms on the benzene ring are replaced by a nitro group, NO2, nitration occurs. Nitrobenzene is widely used in the production of aniline. It is also used in the production of engine and machine lubricants. It is also used in dyes, pesticides, drugs, and synthetic rubber.




