Table of Contents
Introduction
Integrated rate law: The differential rate equation is an equation that represents the rate of reaction’s dependence on the concentration of reacting species. The slope of the tangent at each point in the concentration-time graph represents the instantaneous rate of reaction. As a result, determining the rate of reaction from a concentration-time graph is extremely challenging. As a result, we integrated the differential rate equation to derive a relationship between concentration and rate constant at various places. The integrated rate equation is the name for this equation. We see distinct integrated rate equations for reactions of different orders.
A brief outline
The rate laws we’ve examined so far relate reaction rates to reactant concentrations. The second variant of each rate law that connects the concentrations of reactants and time can also be determined. Integrated rate laws are what they’re called. An integrated rate law can be used to estimate the time required for a reaction to proceed to a particular extent or to quantify the amount of reactant or product present after a length of time. An integrated rate law, for instance, is used to estimate how long a radioactive material must be held earlier its radioactivity decays to a benign level.
The differential rate law for a reaction can be integrated with respect to the time using calculus to yield an equation that connects the amount of reactant or product existing in a reaction mixture to the reaction’s elapsed time. Depending on the intricacy of the differential rate law, this method can be either simple or highly difficult. We’ll concentrate on the corresponding integrated rate laws for first-, second-, and zero-order reactions for the sake of discussion.
Important concepts
The integrated rate equation for the zero-order reaction
In a zero-order reaction, the reaction rate is characterized by the accumulation of reactants to the zeroth power. Zero-order reactions are extremely uncommon. Thermal breakdown of HI on a gold surface, the disintegration of gaseous ammonia on a heated platinum surface, and other zero-order processes are examples. The following is a general formula for a zero-order reaction having to rate constant k:
A → B
Rate = – [dA/dt] = k [A]0
-[dA/dt] = k
=> d[A] = -k dt
Integrating both the sides:
⇒ [A] = -kt + c………………………… (1)
Where, c= integration constant
At time, t=0, [A] = [A0]
Putting the limits used from equation (1) we get the value of c,
⇒ [A0] = c
With the value of c in equation (1) we get,
=> [A] = -kt + [A0]
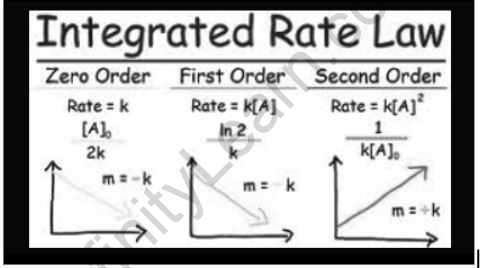
For zero-order reactions, the above equation is known as the integrated rate equation. The above equation can be seen as a straight-line equation, with the concentration of the reactant on the y-axis and time on the x-axis. The value of the rate constant, k, is represented by the slope of the straight line.
The integrated rate equation for the first-order reaction
The rate of reaction in a first-order reaction is determined by the first power of the reactant concentration. First-order reactions include the natural and controlled radioactive decay of unstable nuclei. The following is a general equation for the first order of reaction with rate constant k:
A → B
Rate = – d[A]/dt = k[A]
d [A]/[A] = -k dt
Integrating both the sides:
=> ln [A] = -kt + c………………. (2)
Where c= constant of integration,
At time, t=0, [A] = [A]0
Putting the limits from equation (2) we get the value of c
=> ln [A]0 = c
Using the value of c in the above equation we get,
=> ln [A] = -kt + ln [A]0
The equation above can be displayed as a straight line on the y-axis with ln[A] on the y-axis and time (t) on the x-axis. The rate constant, k, is equal to the negative slope of this straight line.
The integrated rate law for a second-order reaction
The differential rate law condition can be communicated as continues on account of one-second order reactant shaping a specific product in a chemical response:
-d[R]/dt = k[R]
This differential rate equation should be rearranged as follows to acquire the coordinated rate condition:
d[R]/[R]2 = – kdt
The accompanying condition is acquired by coordinating on the two sides while considering the adjustment of reactant fixation between time 0 and time t.
ᶴ[R]t[R]0 d[R]/[R]2 = -kᶴt0 dt
We can find the accompanying from the power rule of reconciliation:
ᶴ dx/x2 = -1/x + C
Where C is the Integration Constant. The accompanying condition can now be gotten by applying the power rule to the past condition.
1/[R]t – 1/[R]0 = kt
Which is the subsequent request response’s required coordinated rate articulation.
Crack NEET with Result-Oriented Learning Program from Infinity Learn
FAQs
The half-life period of a reaction is the time it takes for half of the reactions to complete or for the concentration of the reactant to be lowered to half of its original value.
In a first-order reaction, the half-life time remains constant when the starting concentration of the reactants is doubled. Because the half-life of a first-order reaction is independent of the reactant's initial concentration.
The concentrations of the reactants in a chemical reaction are expressed as a function of time using integrated rate equations. As a result, rate equations may be used to determine how long a certain percentage of the reactants will be consumed in a chemical process. It's worth noting that integrated rate equations for reactions of different orders differ. How long does it take for a half-life to occur?
When the initial concentration of the reactants is doubled, what occurs to the half-life period of a first-order reaction?
What do Integrated Rate Equations mean?
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Ultimate Learning App – Infinity Learn.