Table of Contents
Introduction
Collision theory definition: According to collision theory, only a specific number of collisions between acceptable reactant particles with the correct orientation result in a detectable or noticeable change; these successful modifications are referred to as successful collisions. At the point of impact, effective collisions would have enough energy, also known as activation energy, to break pre-existing bonds and form all new ones. As a result, the reaction’s products are formed. More collisions and thus more successful collisions result from an increase in the concentration of the reactant. The average kinetic energy of the molecules in a solution increase as the temperature rises, increasing the number of collisions with sufficient energy. Max Trautz and William Lewis proposed collision theory in 1916 and 1918, respectively.
A brief outline
When a catalyst is present in the collision between the reactant molecules, the chemical change requires less energy, and thus more collisions have sufficient energy for the reaction to occur. As a result, the reaction rate accelerates. Chemical kinetics and collision theory are intrinsically tied. Diffusion regulates collisions in diluted gas or liquid solutions rather than direct collisions, which may be computed using Fick’s equations of diffusion.
When diffusion takes control of the collision frequency, i.e., when the direct collision between the two molecules no longer dominates, the preceding equation is no longer valid for a diluted solution in the gas or liquid phase. As a result, the pace of a chemical reaction is equal to the frequency of effective collisions, according to the collision theory. Although atomic or molecular collision frequencies can only be estimated with some accuracy for gases, the collision theory’s use is confined to gas-phase reactions.
Important concepts
Collision theory of bimolecular reaction
If ZAB Products is the collision frequency and ‘f′ is the percentage of molecules whose collisions are effective for an elementary bimolecular reaction, A+B, then clearly,
Rate of reaction = dx/dt = ZAB × f……. (i)
In addition, according to the kinetic theory, the fraction of molecules with energy greater than a specified value, E, at a given temperature T, is provided by the Boltzmann factor, i.e.,
f=e–E/RT………(ii)
Now, as the rate of a reaction hinges upon the stimulation energy, Equation (ii) may be inscribed as
f = e–Ea/RT
Replacing this value in equation (i), we get
Rate =ZAB. e–Ea/RT
As the rate of reaction is rightly related to rate constant, k we can similarly write,
k=ZAB.e–Ea/RT
For reactions involving atomic species or simple molecules, this equation properly predicts the values of the rate constant. When the reactant molecules are complex, however, large variations occur. Even if the colliding molecules have more energy than the threshold energy, they may not be oriented properly at the time of contact. It’s possible that no bonds in the reactant molecules will break, and no new bonds will form to generate product molecules.
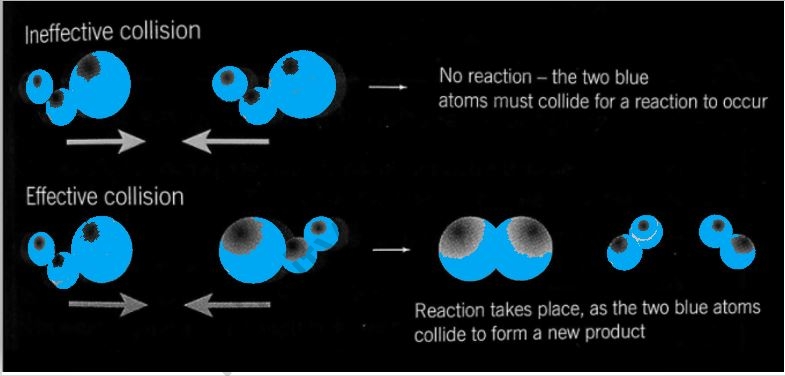
As a result, for a collision to be efficient, the colliding molecules must have more energy than threshold energy and must be oriented correctly. For example, depending on whether bromomethane and alkali have the right orientation at the time, the reaction may or may not result in the creation of methanol.
Collision theory postulates:
a) For any chemical reaction to take place, the molecules of reactants must clash.
b) The reactant molecules’ collisions are fully elastic in nature.
c) Not all collisions result in the production of a product. Only those collisions in which the reactant molecules have a certain amount of energy, known as ‘activation energy,’ result in the production of a product. Effective collisions are a type of collision that occurs when two objects collide.
d) Reactant molecules are assumed to be hard rigid spheres with only transitional energy.
e) For the reaction to take place, the colliding molecules must be appropriately aligned. Effective collisions between improperly orientated molecules take substantially more energy than collisions between properly aligned ones.
f) The number of collisions per cc per second influences the rate of reaction.
Merits of collision theory
1) It is simple to use and allows for quick calculation of reaction speeds.
2) It effectively explains the impact of temperature on response rates.
3) It provides a qualitative explanation for gaseous unimolecular reaction rates.
4) When simple molecules are involved in a gaseous bimolecular process, the rate constant found experimentally agrees perfectly with that. Collision theory was used to calculate the results.
Significance of concept of collision theory in NEET exam
Since the weightage for the areas is very low, the compound energy part has a weightage of around 3.33 percent in throughout the years. This demonstrates that the all out number of inquiries from this subject will be around 1 or 2 and will be worth 4 focuses. The collision thoery and its conditions is regularly expected topic in the chemical kinetics chapter
FAQs
The molecules of reactants are considered to be hard spherical in the collision theory, and reactions are presumed to occur solely when these spheres (molecules) impact each other. It is not necessary for all collisions to result in the synthesis of products; the activation energy and appropriate orientation of the reacting molecules dictate the conditions for a collision that leads to the formation of products.
The reacting species in a chemical reaction can only produce products if they come into contact with one another or crash with one another. It isn't required for all accidents to result in goods. Effective collisions are the collisions between reacting species that result in the product.
The extra amount of energy that must be supplied to the reactant molecules in their general energy state to make their energy equivalent to threshold energy is known as activation energy. What is collision theory and how does it work?
What is the variation between a successful and an unsuccessful collision?
What is the activation energy, and how does it work?