Table of Contents
Table of Contents
- Mole – Definition (Chemistry)
- Summary
- Did You Know?
- What’s Next?
In the previous segment, we learned about molecular mass and formula unit mass. In this segment, we will learn about the concept of a mole.
What is a Mole?
Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
1 mole = 6.022?1023 atoms or molecules or other such smaller particles. Let us see a few examples to understand the concept of mole better.
One mole of hydrogen
1 mole of hydrogen =6.022?1023atoms of hydrogen.
One mole of water
1 mole of pure water = 6.022?1023 molecules of water.
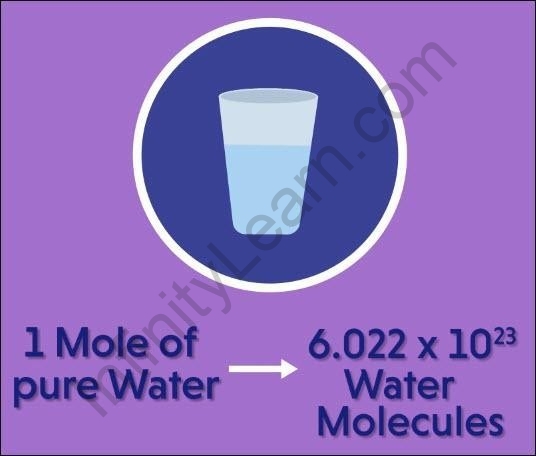
One mole of pure water
Now, if we have 3 moles of water, the number of water molecules increases 3 times.
Three mole of water
3 mole of pure water = 3?6.022?1023 molecules of water




