Table of Contents
The periodic table elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (A) are a group of five or six chemically related elements (At). Ts, a man-made element, has the potential to be a halogen. This group is known as group 17 in modern IUPAC nomenclature. The term “halogen” means “salt former.” When halogens react with metals, they produce a number of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide, and potassium iodide. The halogen group is the only periodic table group that contains elements in three of the four main states of matter at standard temperature and pressure. All halogens react to form acids when they are bonded to hydrogen. The vast majority of halogens come from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are frequently used as disinfectants. Organobromides are the most common type of flame retardant, whereas elemental halogens are hazardous and can be toxic. Nonmetals include the halogens fluorine, chlorine, bromine, and iodine, and the chemical properties of the two heaviest group 17 members have yet to be determined. The chemical bond energy of halogens decreases from top to bottom of the periodic table column, with fluorine deviating slightly. It has the highest bond energy in compounds with other atoms, but the bonds within the diatomic F2 molecule are extremely weak. This means that as one moves down group 17 in the periodic table, the reactivity of elements decreases due to the increasing size of the atoms.
On the periodic table, halogens are a group of elements. It is the only element group that contains elements that can exist at room temperature in three of the four major states of matter: solid, liquid, and gas. The word halogen means “salt-producing” because halogens react with metals to produce many important salts. In fact, halogens are so reactive that they do not exist as free elements in nature. Many, on the other hand, are frequently found in conjunction with other elements. Here’s an explanation of what these elements are, where they are on the periodic table, and what they are used for.
Halogens are classified as Periodic Table Group VIIA, or Group 17 in IUPAC nomenclature. The element group is a nonmetal subset. They are arranged vertically on the right side of the table. Halogens are nonmetals. Fluorine and chlorine are gases at room temperature, whereas bromine is a liquid. Iodine and astatine are solids. Fluorine, astatine, and other halogens are extremely reactive, with decreasing reactivity from fluorine to astatine. Halogens do not exist in their elemental form in nature. Astatine isotopes have radioactive half-lives and have short half-lives.
Overview
When an acid contains oxygen (referred to as an oxoacid), the suffixes –ous and –ic are used to represent the lower and higher number of oxygens in the acid formula. Hydrogen, oxygen, and other elements combine to form oxyacids. Oxoacids are acids made up of hydrogen, oxygen, and another element. The Halogen Family Oxoacids are discussed in this topic. Fluorine, chlorine, bromine, iodine, and astatine are all members of Group 17. They are referred to collectively as halogens, which means salt producers. The members of this group are eerily similar. There is a consistent gradation in physical and chemical properties as we move down the group. The only radioactive element in the group is astatine. Their valence shell contains seven electrons (ns2np5), one fewer than the closest noble gas configuration. Halogens are small in size due to their effective nuclear charge. As a result, they have a lower chance of losing electrons and a higher chance of gaining an electron to complete their octet. Halogens produce a number of oxoacids (they are acids that contain oxygen in the acidic group).
Fluorine, chlorine, bromine, iodine, and astatine are the elements in Group 17 from top to bottom. Because they produce salt, they are referred to as halogens. The members of this group are very similar to one another. They have a consistent set of physical and chemical properties. The valence shell of each of these elements contains seven electrons.
Their electronic setup is ns2 np5. If the acid contains oxygen (referred to as an oxoacid), the suffixes –ous and –ic are used once more to indicate the lower and higher number of oxygens in the acid formula. Oxyacids are formed when hydrogen, oxygen, and other elements combine. Oxyacids are acids that contain hydrogen, oxygen, and another element.
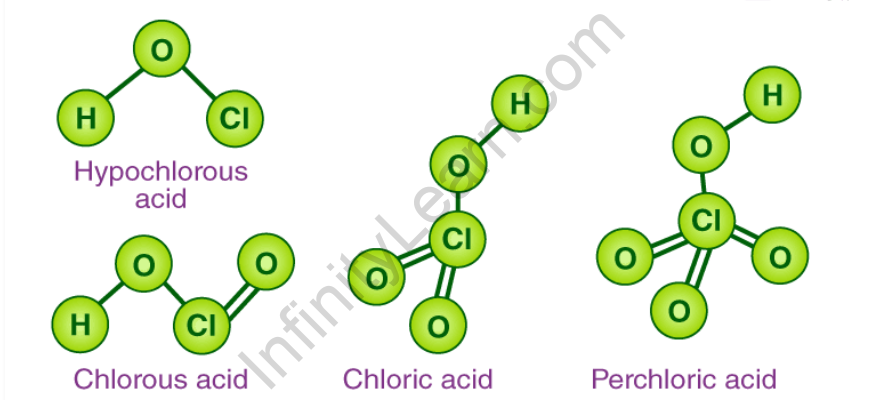
Oxoacids of halogens structure
The oxoacids’ central atom is sp3 hybridised. Every oxoacid has a single X-OH bond. In contrast, most oxoacids contain X=O bonds. In nature, this double bond between oxygen and halogen is known as d pi-pi. The first member of the oxoacid series has high acidic strength. This is due to the halogen atom’s high electronegativity and small size. The acidic strength increases as the number of halogens oxidised increases.
Acidic character of oxoacids of halogens
The acid strength of a compound is determined by how easily it can donate/let go of the hydrogen atom present in it as a proton. The ease with which this occurs is determined by the electronegativity of the other atoms bonded directly to or near hydrogen. Acid strength is now determined by the ease with which a hydrogen atom can leave as a proton (H+). To achieve this, the partial charge on hydrogen must be as positive as possible. In other words, the oxygen must be able to attract the OH bond’s shared pair of electrons. To make this happen as easily as possible, the halogen atom’s electronegativity should be as high as possible, so that it pulls the shared pair of electrons of the XO bond towards itself, making oxygen partially electron-deficient, resulting in it pulling the shared electrons of the OH bond towards itself, making the liberation of the H+ ion easy.
As a result, the acid strength increases as the electronegativity of the halogen atom increases. As we know, atomic size increases as we move down a group, which reduces the nuclear pull (attraction between the nucleus and the electrons) as electrons move further away from the nucleus. This results in a decrease in electronegativity as one moves down the group. As a result, the acid strength of halogen oxoacids decreases as we move down the group.
Acidity order of oxoacids of halogens
The acidic strength of oxoacids with the same halogen atom oxidation number decreases with increasing atomic number, i.e., with decreasing electronegativity of the atom. The electronegativity of the attached halogen atom to oxygen decreases in the order Cl>Br> I. As a result, the tendency of the oxygen atom to withdraw electrons towards itself decreases from Cl to Br to I. As a result, the proclivity to pull electrons from hydrogen decreases.
As a result, releasing $mathrmH+ion from HClOwill be easier than HBrO.Similarly, releasing H+ion from HBrO will be simpler than from HIO.
Thus, the acidic strength decreases in the following order: HClO>HBrO>HIO
FAQs
What exactly are oxoacids?
Oxoacids are acids that contain oxygen. It is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen molecule bound to oxygen that can separate to form the acid's H+ cation and anion.
What is the one thing that all oxyacids have in common?
Oxygen is present in all oxyacids. It is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen molecule bound to oxygen that can separate to form the acid's H+ cation and anion.




