Table of Contents
Chloroform, also known as trichloromethane, is an organic compound with the chemical formula CHCl3.It is a colourless, strong-smelling, dense liquid that is mass-produced as a precursor to PTFE. It’s also a precursor to a number of refrigerants. The total global flux of chloroform through the environment is approximately 660000 tonnes per year[11], with approximately 90% of emissions coming from natural sources. Many types of seaweed produce chloroform, and it is believed that fungi produce chloroform in soil. Although the mechanism is unknown, abiotic processes are thought to contribute to natural chloroform production in soils.
Chloroform readily volatilizes from soil and surface water and degrades in the air to produce phosgene, dichloromethane, formyl chloride, carbon monoxide, carbon dioxide, and hydrogen chloride. In the air, its half-life ranges from 55 to 620 days. Water and soil biodegradation is slow. Chloroform does not bioaccumulate significantly in aquatic organisms. It is one of the four chloromethanes and a trihalomethane. When inhaled or ingested, it has potent anaesthetic, euphoriant, anxiolytic, and sedative properties. This article discusses and explains the Chloroform Formula, as well as its structure. Chloroform, also known as trichloromethane, is an organic compound that is widely used in the pharmaceutical and chemical industries. Because of its anaesthetic properties, chloroform is widely used in medicine. It is also used as a solvent in the production of Teflon, the synthesis of pesticides, resins, and other chemicals, and the production of the Freon refrigerant R-22. Inadvertently, the haloform reaction can also occur in domestic settings.
Inside reactions, hypochlorite bleaching generates halogenated compounds, the main byproduct being chloroform. Chloroform can be produced when sodium hypochlorite solution (chlorine bleach) is mixed with common household liquids such as acetone, methyl ethyl ketone, ethanol, or isopropyl alcohol, in addition to other compounds such as chloroacetone or dichloroacetone.
Overview
Trichloromethane molecules are made up of three chlorine atoms attached to one carbon atom. Polyhalogenated compounds are organic compounds that have two or more halogen atoms attached to them. Because of the high reactivity of halogens, polyhalogenated organic compounds are a very important class of chemicals. Chloroform is also a member of this family of compounds. Despite the fact that these compounds have been shown to have negative effects on the environment and the human body, industries continue to use them due to their high utility and wide range of applications. Chloroform is an organic chemical compound that was originally used as an anaesthetic. CHCl3 is the chemical formula. It is a colourless, sweet-smelling dense liquid that is mass-produced. Chloroform may be released into the atmosphere as a byproduct of the chlorination of drinking water, wastewater, and swimming pools. Chloroform is an industrial chemical that has the ability to act as a lacrimator. It is non-flammable, making it safer to work with than ethanol. Trichloromethane is a solvent for iodine, alkaloids, fats, and a variety of other substances. Chloroform is primarily used in the production of the Freon refrigerant R-22. Because of global warming, the use of R-22 as a refrigerant has been phased out in developed countries, but it remains in high demand in developing countries due to its ease of availability and manufacturing. Since its effects were discovered, it has been used as a prominent anaesthetic during medical surgeries. Because of its anaesthetic properties, this compound is even used by criminals to knock out their victims. Breathing air containing 900 ppm chloroform has been linked to dizziness, headaches, and fatigue.
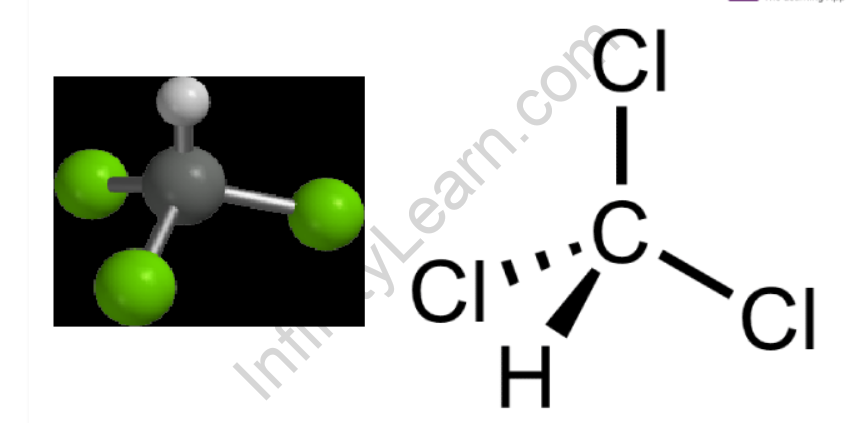
Chloroform has a tetrahedral structure similar to methane, with three hydrogen atoms replaced by three chlorine atoms. Chloroform’s (trichloromethane’s) structure is represented as-
Environment effects of – trichloromethane
Chloroform is a fascinating chemical. It is well-known for its anaesthetic properties and is useful in industry and science laboratories. It is, however, a toxic substance that, when sufficiently concentrated, can cause serious health problems. These issues include organ damage and irregular heartbeats. Inhaling chloroform at high concentrations can depress the respiratory system to the point of death. The chemical is also thought to increase the risk of cancer. Chloroform is abundant in the environment and can be produced both naturally and artificially. Most people are exposed to low levels of the chemical, but some people are exposed to higher levels, particularly those working in certain industries. Fortunately, there are methods for limiting our exposure to the substance.
Trichloromethane is harmful to both the environment and the human body. In terms of atmospheric pollutants, it is not thought to have a significant negative impact. Water-dwelling organisms may be endangered during major accidental spills. If it is spilled on land, it is leached into groundwater, where it can remain for long periods of time. Its bioconcentration in the food chain is unknown, and its small amount is adsorbed to the sediments or soil. In the low tropospheric ozone layer, trichloromethane has negligible photochemical reactivity. It makes no contribution to the photochemical smog. It has a long life in the atmosphere, lasting months, and it travels long distances through the air.
When the concentration of chloroform in the air reaches 900 ppm, it causes fatigue, headache, and dizziness. It is harmful to the central nervous system, the liver, the digestive system, the eyes, and the kidneys. When chloroform is oxidised in the presence of light, phosgene, a highly poisonous gas, is produced. As a result, it is stored in airtight bottles that are dark in colour to avoid contact with air. Because of the negative effects on the environment, the applications and uses of trichloromethane are now overshadowed.
Chloroform trichloromethane
Chloroform, also known as trichloromethane, has the formula CHCl3. It is a clear, colourless, dense liquid with a pleasant odour that is nonflammable at room temperature. It has a sweet flavour but leaves a hot, burning sensation in the mouth and throat. When the liquid comes into contact with the skin, it causes sores. Chloroform in liquid form is a highly flammable substance. This means that at normal environmental temperatures, it has a proclivity to condensate into a vapour.
Chloroform is synthesised for industrial use and is naturally produced from chlorine in the environment. It is also produced by seaweeds and microalgae. Although its non-medical applications are beneficial, the chemical must be handled with caution when used in laboratories and industrial processes.
Fluoro trichloromethane
Trichlorofluoromethane was widely used as a refrigerant at first. Because of its high boiling point (in comparison to most refrigerants), it can be used in systems with low operating pressures, making the mechanical design less demanding than that of higher-pressure refrigerants R-12 or R-22. For fluorine-19 NMR studies, trichlorofluoromethane is used as a reference compound. Trichlorofluoromethane was previously used in the drinking bird novelty, owing to its high boiling point of 23.77 °C (74.79 °F). Dichloromethane, with a boiling point of 39.6 °C (103.3 °F), requires a higher ambient temperature to function. Prior to the discovery of the ozone depletion potential of chlorine in refrigerants and other potentially harmful environmental effects, trichlorofluoromethane was occasionally used as a cleaning/rinsing agent for low-pressure systems.
Effect of trichloromethane
Science’s advancements have provided us with a plethora of things to ponder.
- These products have made our lives easier and raised our standard of living, but some scientific discoveries and products have had negative effects on the human body and the environment.
- The uses and applications of chloroform are now overshadowed by its harsh effects on the environment and the human body, and its use has been significantly restricted in most countries.
FAQ’s
What are the applications of chloroform?
Chloroform is a colourless, volatile liquid that is a trichloromethane derivative with an ether-like odour. Previously used as an inhaled anaesthetic during the surgery, chloroform's main application today is in agriculture, where it is used as a solvent, and especially in the manufacture of refrigerant freon.
What effects does chloroform have on the human body?
Shortness of breath, as well as nose and throat irritation, are common local symptoms of chloroform inhalation. Acute inhalation can cause systemic symptoms like agitation, nausea, and vomiting, as well as ataxia, dizziness, and drowsiness.








