Table of Contents
An isocyanide (also known as isonitrile or carbylamine) is a type of organic compound with the functional group -N≡C. Because it is an isomer of the related nitrile (-CN), the prefix is isocyano. The organic fragment is linked to the isocyanide group via the nitrogen atom rather than the carbon atom. They are used in the synthesis of other compounds as building blocks.
In isocyanides, the C-N distance is 115.8 pm in methyl isocyanide. The angles C-N-C are close to 180°. Isocyanides, like carbon monoxide, have two resonance structures: one with a triple bond between the nitrogen and the carbon and one with a double bond. Although the reactivity of isocyanides reflects some carbene character, at least in a formal sense, the lone pair of nitrogen stabilises the structure and is responsible for the linearity of isocyanides. As a result, both resonance structures serve as useful representations. They are polymerization-susceptible.
Isocyanide is the strangest, most fascinating, and one-of-a-kind functional group in organic chemistry. Its chameleonic nature allows its carbon atom to be the subject of virtually all reactivities in organic chemistry. Indeed, it can act as a nucleophile attacking activated electrophiles, an electrophile being intercepted by various nucleophiles, a carbene involved in formal [4 + 1] cycloaddition, and a radical acceptor to form imidoyl radical reaction intermediates. Finally, the presence of a lone pair on the terminal carbon atom explains its strong metal coordinative properties, which allow for the formation of an infinite number of coordination complexes. Isocyanides are a class of reagents that are extremely versatile and indispensable because the isocyano group can act as both a nucleophile and an electrophile in chemical reactions. Isocyanide chemistry has received a lot of attention and has found a lot of applications in synthetic organic and medicinal chemistry for the construction of structurally interesting and diverse N-heterocycles or peptidomimetics over the last few decades.
Overview
Isocyanide is an organic compound discovered in 1867. This compound has the functional group – N≡C. It is also referred to as Carbylamine or Isonitrile. It is also an isomer of nitrile, i.e., – C≡N., thus the prefix iso. Furthermore, this organic segment is linked to the isocyanide group via the nitrogen atom rather than the carbon atom. Isocyanides are also used as building blocks in the synthesis of other compounds.
Isocyanide, also known as Isonitrile or Carbylamine, is a class of organic mixtures with the subatomic construction R – N+ C, where R is a combining group inferred by the expulsion of a hydrogen atom from an organic compound. Isocyanides are nitrile isomers that were discovered in 1867 but have yet to achieve widespread utility. They are frequently obtained as minor items in the union of nitriles from metal cyanides and natural halogen compounds after being formed from essential amines by treatment with chloroform and salt. They have both incredible and horrifying scents.
A nitrogen molecule is attached to a carbon particle and a R (alkyl group) in an isocyanide formula (also known as isonitriles), with a resonance structure containing a triple bond, producing a carbanion and a positive nitrogen particle. For organic cyanides (R – C≡N), the postfix “isonitrile” or “carbylamine” is commonly used in IUPAC classification. The prefix “isocyano” appears in the names of isocyanides. As a result, the IUPAC names are changed to isocyanomethane, isocyanoethane, isocyanopropane, and so on. The term “carbylamine,” which is occasionally used, contradicts deliberate classification. An amine always has three single bonds, whereas an isocyanide only has one single and one different bond. An amino group is linked to an isocyano moiety in the isocyanamide practical gathering. The priority table is used to determine whether a postfix of isonitrile or a prefix of isocyano is used for terminology. Isocyanides are stable to a stable base (they are regularly produced under unequivocally basic conditions), but they are sensitive to corrosive. Isocyanides hydrolyze to the corresponding formamides in the presence of watery corrosive:
RNC + H2O → RN(H)C(O)H
This reaction is used to destroy musty isocyanide mixtures. In the presence of Lewis and Bronsted acids, some isocyanides can polymerize. In the natural blend, isocyanides participate in a number of multi-component responses of interest, two of which are the Ugi response and the Passerini response.
Methylisocyanide
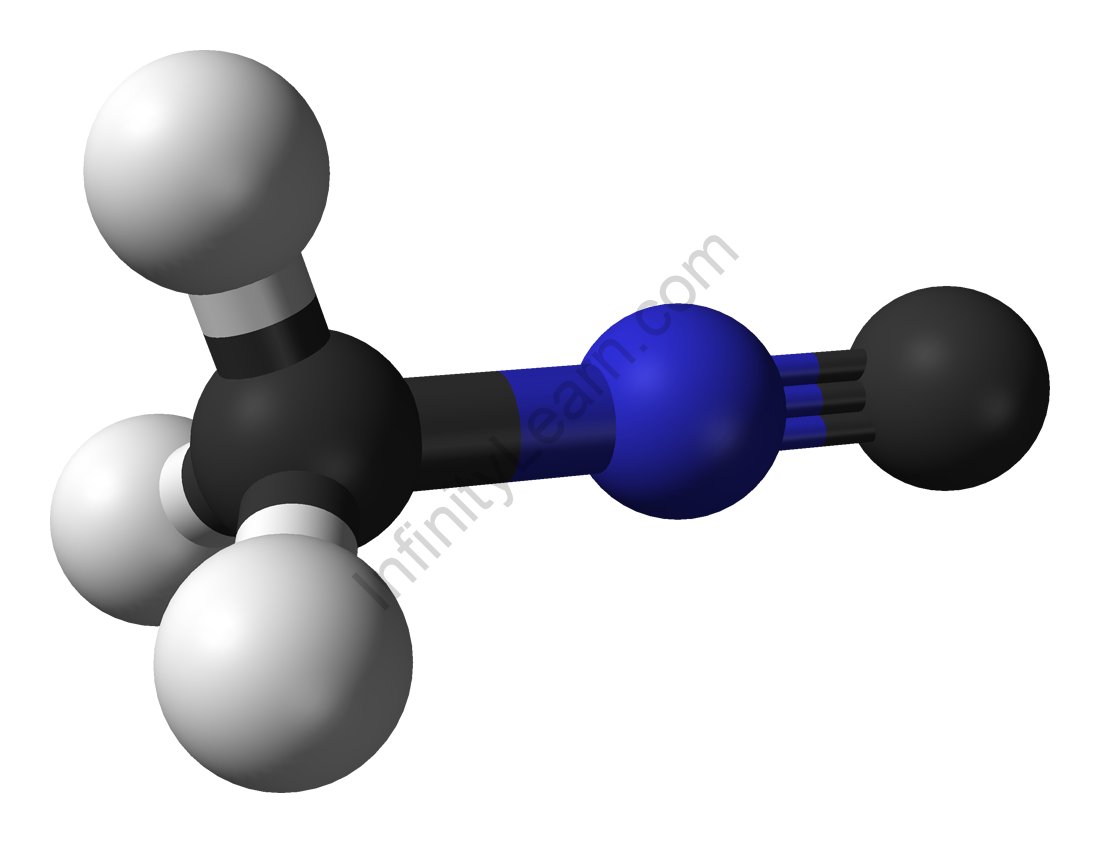
Methyl isocyanide, also known as isocyanomethane, is an organic compound that belongs to the isocyanide family. This colourless liquid is isomeric to methyl cyanide (acetonitrile), but its reactivity differs greatly. Unlike acetonitrile, which has a faintly sweet, ethereal odour, the odour of methyl isocyanide, like that of other simple volatile isocyanides, is distinctly penetrating and vile. Methyl isocyanide is primarily used in the formation of 5-membered heterocyclic rings. As is typical of isocyanides, the C-N distance in methyl isocyanide is very short, 1.158.
A. Gautier first synthesised methyl isocyanide by reacting silver cyanide with methyl iodide. The dehydration of N-methylformamide is a common method for producing methyl isocyanides. Many metal cyanides react with methylating agents to form methyl isocyanide complexes. This type of reactivity has been suggested as being important in the origin of life. Methyl isocyanide can be used to make a variety of heterocycles. It is frequently used in the preparation of transition metal isocyanide complexes.
Cyanide and isocyanide
Isocyanide is an organic compound with a carbon-nitrogen triple bond and an alkyl or aryl group attached to the nitrogen. It is also known as Isonitrile or Carbylamin. The organic fragment is linked to the isocyanide group by the nitrogen atom rather than the carbon atom. They are used in the synthesis of other compounds as building blocks. Isocyanides are nitrile isomers that were discovered in 1867 but never found widespread application. They are commonly obtained as minor products in the synthesis of nitriles from metal cyanides and organic halogen compounds after being prepared from primary amines by treatment with chloroform and alkali. They have incredibly strong and repulsive odours. Isocyanides are useful tools for creating structurally diverse chemical libraries. Isocyanides are a diverse group of metabolites produced by microorganisms on land, marine organisms, and plants.
Cyanides are made up of one nitrogen atom and one carbon atom linked together by a triple bond. Cyanide anion or nitrile anion Cyanide ions are other names for it. Cyanide can be produced by certain algae, fungi, and bacteria. It can also be found in vehicle exhaust, spinach, almonds, tapioca, and other foods. At room temperature, hydrogen cyanide appears as a colourless or pale blue liquid, and at higher temperatures, it transforms into a colourless gas with a bitter almond odour.
Some cyanides, such as potassium cyanide and sodium cyanide, are white powders with a bitter almond odour. Cyanide-containing compounds and cyanide are used in plastics, drug and dye manufacturing, photo development, and other industries. Some industrial processes, such as wastewater treatment and steel production, produce cyanide.

Isocyanide examples
Isocyanides, unlike cyanides, have only common names. Isocyanides are known as Alkyl isocyanides in the common system. The parent alkane is selected, and the ending ‘e’ is replaced by ‘yl’ followed by the suffix ‘isocyanide.’ CH3NC, for example.
Alkane parent: Methane
Methyl isocyanide (common name)
They are also known as alkyl carbylamines. CH3NC, for example.
Alkane parent: Methane Methyl carbylamine is its common name.
| Structural Formula | Common Name |
| CH3 NC | Methyl isocyanide or Methyl carbylamine |
| CH3 CH2 NC | Ethyl isocyanide or Ethyl carbylamine |
| CH3 CH2 CH2 NC | Propyl isocyanide or Propyl carbylamine |
| C6H5 NC | Phenyl isocyanide or Phenyl carbylamine |
Because of their small size, unique reactivity, and good biocompatibility, isocyano functionalities are considered promising bioorthogonal handles. There must be a suitable counterpart for a bioorthogonal reaction to occur, allowing for good reaction kinetics and ideal products. As a result of the discovery that large sterically hindered tetrazines can form stable products rapidly after conjugation with primary isocyanides, the use of isocyanides in biological systems increased rapidly. Chlorooxime has recently been discovered to be another complementary counterpart for bioorthogonal ligation with isocyanides, thus expanding the repertoire of bioorthogonal chemistry involving isocyanides.
FAQ’s
What are the Consequences of Isocyanide Exposure?
Exposure to isocyanates can cause substance bronchitis and pneumonitis. An isocyanate reaction frequently includes hacking, chest tightness, windedness, sickness, regurgitation, eye and skin aggravation, gastric torment, and loss of cognizance.
Isocyanates are commonly found in what types of products?
Isocyanates are commonly found in automobile paints, froth pads, sleeping cushions, vehicle seats, froth protection, froth bundling materials, under-cover cushioning, polyurethane elastic, and glues.
What is the Isocyanide General Scheme?
Isocyanides are extremely poor electrophiles, able to react only with solid nucleophiles such as organometallic compounds. The electron-withdrawing effect of the isocyano group in aliphatic isocyanides, like the ortho-methyl bunch in fragrant isocyanides, increases the acidity of the a-C-H bond group.






