Table of Contents
Phenols are chemical compounds that have a benzene ring that is linked to a hydroxyl group. They are sometimes referred to as carbolic acids due to their extreme acidity. Phenoxide is formed when phenols react with active metals such as sodium and potassium. This interaction of phenol with metals suggests that it is acidic. Benzonal is the IUPAC term for phenol. The phenol can be substituted using either the Ortho meta para or the numbering system. The parent molecule should be referred to as phenol in any situation. Cresols are common names given to some phenols, such as phenols.
Phenols, like aqueous sodium hydroxide, react to form phenoxide ions. This suggests that phenols have a greater acidity than alcohol and water molecules.
The Acidity of Phenols: An Explanation
The propensity of phenols to lose hydrogen ions and produce phenoxide ions accounts for their acidity.
The sp2 hybridized carbon atom of the benzene ring connected directly to the hydroxyl group works as an electron-withdrawing group in a phenol molecule.
The electronegativity of this sp2 hybridized carbon atom of a benzene ring connected directly to the hydroxyl group is greater than that of the hydroxyl group.
The electron density on the oxygen atom lowers due to the increased electronegativity of this carbon atom in contrast to the hydroxyl group connected.
The reduction in electron density raises the polarity of the O-H bond, resulting in increased phenol ionization.
As a result, the phenoxide ion is generated. The formation of the phenoxide ion is stabilized by the delocalization of the negative charge caused by the resonance in the benzene ring.
Phenoxide ion is more stable than phenols because charge separation occurs during resonance in the case of phenols.
The delocalization of negative charge is explained by the resonance structures of phenoxide ions. The acidity of phenols rises in the presence of an electron-withdrawing group in the case of substituted phenols. This is all because of the phenoxide ion’s stability. The acidity of phenols rises much more if these groups are linked at ortho and para locations. This is because the negative charge in the phenoxide ion is primarily delocalized in the ortho and para locations of the connected benzene ring. The acidity of phenols, on the other hand, diminishes in the presence of electron-donating groups because they prevent the production of phenoxide ions.
Reaction Showing Acidic Nature Of Phenol
The acidic character of phenol can be represented by the two reactions listed below:
- When phenol interacts with sodium hydroxide, sodium phenoxide and water are produced as byproducts.
- Phenol interacts with sodium to form sodium phenoxide, which releases H2.
The acidity of phenol is more than that of ethanol. This is because after losing a proton, the phenoxide ion undergoes resonance and gets stabilized whereas the ethoxide ion does not.
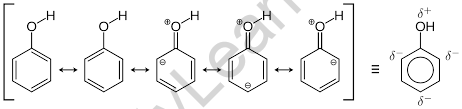
Resonance of Phenol
The ion or the molecule is said to have resonance when more than one Lewis structure can be drawn.
Resonance is a notion in which electrons are delocalized across three or more atoms of a compound or molecule, and the Lewis structure of that molecule cannot be represented as a single, uncomplicated structure.
Take note that three of the four contributing structures have a positive charge on the oxygen atom of the molecule. As a result, the real hybrid structure must have some positive charge. Due to the fact that oxygen is an electronegative element, the electrons in the oxygen-hydrogen bond orbital gravitate to the oxygen atom, resulting in partly positive hydrogen.
When a hydrogen ion is lost to a base, it produces a phenoxide ion that is totally resonance stabilized. Also, notice how the phenoxide anion is formed when hydroxy hydrogen is removed by a base. This anion is resonance stabilized by the delocalization of an electron pair across the molecule, as shown by the contributing structures.
Properties of Phenol as an Acid
A number of the features of phenol, when combined with various solutions, are described below.
-
Considering indicators
The pH of a typical dilute phenol solution in water ranges between 5 and 6, depending on concentration. It signifies that a very dilute solution is not acidic enough to eventually turn a litmus paper crimson. Litmus paper, on the other hand, will be blue at pH = 8 and red at pH = 5. If there is anything in between, it will be represented as a shade of “neutral.” When phenol interacts with sodium hydroxide solution, it produces a colourless solution containing sodium phenoxide. The strongly basic hydroxide ion in the sodium hydroxide solution eliminated the hydrogen ion during this reaction.
-
With Sodium Carbonate or Sodium Hydrogen Carbonate
Phenol is insufficiently acidic to react with any of these. Carbonate and hydrogen carbonate ions, on the other hand, are not solid enough to extract a hydrogen ion from phenol. Unlike most acids, phenol does not emit carbon dioxide when combined with another. Furthermore, this lack of reactivity is highly beneficial. We can also identify phenol for the reasons indicated below.
- It is water-soluble but not soluble.
- It must be acidic because it frequently interacts with sodium hydroxide solution to form a colourless solution.
FAQs
What causes phenol to be acidic?
Acidity in phenols is caused by the release of H+ ions from the hydroxyl group. As the OH group is involved in resonance, a proton is released, and the oxygen receives a partial positive charge. This allows the H+ ions to readily exit, resulting in phenols being a Bronsted acid. Because the ion phenoxide is stabilized by resonance, phenols are more acidic than their alcohol counterparts. This facilitates the elimination of H+ ions in phenols.
What are the applications of phenol?
Phenols and their derivatives are employed in a variety of applications, including the production of pharmaceuticals in the pharmaceutical sector, antiseptics in soaps and disinfectants, the manufacture of azo- colours, the production of picric acid, and as ink preservatives. They are also utilized as intermediates in industrial synthesis, and it was the first surgical antiseptic used in domestic items.
What is the chemical structure of phenol?
Because phenol has a ring structure, the carbon atom in the Ring (with alternating double bonds) is sp2 hybridized. The –OH group is connected to the aromatic ring's sp2 hybridized carbon atom. As a result, the C–O bond is formed when an overlapping sp3 orbital of oxygen and an sp2 hybridized orbital of carbon occur in the aromatic ring.








