Table of Contents
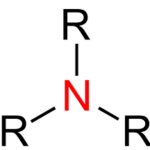
Amines are derived from ammonia (NH3). In other words, we will simply say that amines are derivatives of ammonia. We study amines in chemistry, and they are basically classified as functional groups or organic nitrogen compounds that contain a nitrogen atom with a lone pair.
Some of the present amines include alkaloids found in certain plants; catecholamine neurotransmitters (i.e. dopamine, epinephrine, and norepinephrine); and a selected chemical mediator, histamine, existing in most animal tissues. Common samples of amines include amino acids, trimethylamine, and aniline. Meanwhile, inorganic ammonia compounds like mono chloramine (NClH2) also are called amines.
Amines naturally occur in hormones, vitamins, proteins, etc. they will even be prepared synthetically
Nomenclature of Amines
In chemistry, the names of the compounds that are globally accepted are consistent with the rules given by IUPAC for the nomenclature of organic compounds. The naming of aliphatic amines is completed by prefixing the alkyl to amines and thus the names of aliphatic amines are of the shape of alkylamine. For example, CH3NH2 is known as methylamine (alkyl part + amine =methylamine). Prefixes like di and tri are appended before the names of the alkyl when two or more identical groups are present.
If quite one amino is present within the amine then the parent chain and therefore the position of amino groups is identified by numbering the carbon atoms within the parent chain. The numbering is completed in such a way that the atoms bearing the –NH2 groups get rock bottom numbers. Prefixes alongside the amounts are then wont to denote the number of amino groups and their position within the molecule. For example, H2N-CH2-CH2-NH2 is known as ethane 1, 2-diamine.
If –NH2 group is attached to a benzene formula then it’s called arylamines.
One of the only samples of arylamine is C6H5NH2. It’s commonly referred to as aniline which is additionally an accepted IUPAC name. Once we name arylamines consistent with the rules given by IUPAC then ‘e’ of the arene is replaced by amine for instance C6H5-NH2 is known as benzene amine.
The oldest and most typically used methane series naming system is to classify every cluster connected to the gas atom so add the ending amine, as in methylamine, CH3NH2; CH3CH2NHCH2CH2CH3; N-ethyl-N-propylamine (or ethyl(propyl)amine) and tributylamine, (CH3CH2CH2)3N.
Any or more of the listed groups are in alphabetical order; Ns or inner brackets are wont to illustrate what groups are sure to nitrogen instead of to every other.
Numerous fragrant amines and most cyclic amines have unimportant (non-deliberate) names (e.g., aniline) which may be utilized as a parent to assign the opposite associated, as in N, N-dimethylaniline.
An alternative method substitutes a hydrocarbon name’s terminal -e with the suffix-amine for the functional group −NH2. the most important group is chosen because the parent of secondary and tertiary amines, and therefore the other groups are designated as substituents.
Examples of amines
- CH3− CH2 − CH (NH2) – CH3 (2- Butanamine)
- CH3− CH2 − CH (NHCH3) – CH3 (N – Methyl -2- Butanamine)
- H2N − CH2– CH2– OH (2- Methanol)
Glucosamine
Although glucosamine isn’t used for acute low back pain, it’s widely advertised to the overall public as a treatment for osteoarthritis. Glucosamine is of course found within the body, especially in cartilage, tendons and ligaments, and must be synthesized by the body because significant amounts aren’t found within the diet. Its active form,d-glucosamine, is employed within the manufacture of glycosaminoglycan, a precursor to cartilage tissue.
When studies of sound methodological quality were used, the review did not find any difference between glucosamine and placebo with regards to pain and changes within the Western Ontario and McMaster Osteoarthritis Index (WOMAC) function score. It still remains uncertain whether the 2 salts of glucosamine available, sulphate and hydrochloride, are equally active. Studies thus far have also been relatively short (2–3 months), and any long-term benefits are still uncertain.
Catecholamine
A catecholamine may often be a monoamine.
Monoamine is a compound that usually features a catechol and a side-chain amine(i.e benzene with two hydroxyl side groups next to each other).
Catechol is often either a free molecule or a substituent of a bigger molecule, where it represents a 1,2-dihydroxybenzene group.
Catecholamines are derived from the amino alkanoic acid tyrosine, which springs from dietary sources also as synthesis from phenylalanine. Catecholamines are water-soluble and are 50% sure to cause plasma proteins in circulation.
Some catecholamines are
- epinephrine (adrenaline),
- norepinephrine (noradrenaline)
- dopamine.
The release of the hormones epinephrine and norepinephrine from the medulla of the adrenal glands is a component of the fight-or-flight response.
Tyrosine is made from phenylalanine by hydroxylation by the enzyme phenylalanine hydroxylase. Tyrosine is additionally ingested directly from dietary protein. Catecholamine-secreting cells use several reactions to convert tyrosine serially to L-DOPA then to dopamine. counting on the cell type, dopamine could also be further converted to norepinephrine or may be further converted to epinephrine.
Various stimulant drugs (such as a variety of substituted amphetamines) are catecholamine analogues.
FaQs
What are samples of amines?
Amino acids, biogenic amines, trimethylamine, and aniline are essential amines; see Category: Amines for an inventory of amines. Ammonia inorganic derivatives also are referred to as amines, for instance, mono chloramine (NClH2). The amino is named the substituent -NH2.
Why amines are basic in nature?
Owing to the presence of one pair of electrons on nitrogen, amines are fundamental in nature. By comparing the availability of pairs of electrons on nitrogen, amine basicities are often contrasted with ammonia supported.






