Table of Contents
Introduction
- It is said that assuming one could appraise how much sulphuric corrosive delivered in a country, then, at that point, that data is all that could possibly be needed to assess the modern development of that specific country.
- This is on the grounds that sulphuric corrosive is a fundamental natural substance for nearly all that is economically made.
- It is ordinarily utilized in manure producing, oil refining, mineral handling and it is even utilized in wastewater handling.
- A few different purposes incorporate homegrown acidic channel cleaners, as electrolytes in lead-corrosive batteries, as a getting dried out specialist, and so on
- More or less, sulphuric corrosive is a solid mineral corrosive portrayed by its solid getting dried out and oxidizing nature.
- It has a compound equation H2SO4.
This corrosive is dismal with an impactful smell.
It is solvent in water and deliveries heat on contact.
The sub-atomic design of sulphuric corrosive is addressed beneath as a line-wedge-run structure.
Assembling of Sulphuric Acid By Contact Process
- By and large, there are multiple ways of assembling sulphuric corrosive.
- Every one of them shifts in the work, cost, and virtue of the sulphuric corrosive that is delivered.
- Nonetheless, the most well-known process among these is the contract cycle.
The production of sulphuric corrosive utilizing the contact cycle includes four stages. These incorporate;
- Extraction of sulfur.
- Arrangement of sulfur dioxide.
- Change of sulfur dioxide to sulfur trioxide.
- Transformation of sulfur trioxide to sulphuric corrosive.
Extraction of Sulfur
Unadulterated sulfur is expected for the creation of sulfur dioxide gas.
There are many hotspots for separating sulfur.
The main one among these is the recuperation from gaseous petrol and oil.
The natural or mineral pieces of these are eliminated to acquire sulfur.
Aside from the extraction of unadulterated sulfur, there are not many ways by which sulfur dioxide can be separated. Metal refining is one of them.
Numerous metal minerals happen as sulfides in the dirt.
Later they are cooked to acquire their separate oxide and sulfur dioxide.
Readiness of Sulfur Dioxide
Sulfur dioxide can be ready by consuming unadulterated sulfur within the sight of abundant air.
This causes the development of dioxide of sulfur.
The course of sulfur dioxide planning is as per the following:
- Liquid sulfur is siphoned into the fixed atomizer.
- This causes the arrangement of atomized sulfur.
- This atomized sulfur is applied to the hot heater.
- Air preheated and dried utilizing a sulphuric corrosive dehydrator and additionally applied to the hot heater.
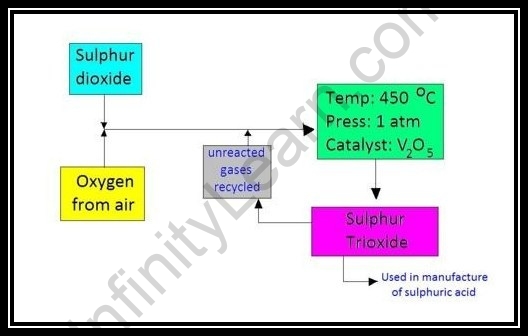
Fundamental Conditions for Obtaining Maximum Sulphuric Acid Yield
Fixation Consideration
- A 1:1 extent of sulfur dioxide and oxygen.
- From the situation, we can see that there is consistently an abundance in oxygen assuming we utilize this proportion.
- As per Le’ Chatelier’s guideline, on the off chance that how much reactant turns out to be high, the balance will tip towards the item side.
- Since how much oxygen is generally higher on the reactant side, the response will yield sulfur trioxide.
Temperature Consideration
This response is exothermic in nature as displayed:
2SO2(g) + O2(g) ⇌ SO3 (g) ΔH = – 196 KJ mol-1
Subsequently, as per Le’ Chateliers’ standard, a lower temperature yields more sulfur trioxide.
Notwithstanding, it’s anything but a plausible method for decreasing the temperature excessively low.
Pressure Consideration
- From the situation, we can see that on the reactant side, there are three reactant particles and on the item side just two.
- That implies the use of overabundance pressure leans toward the development of more sulfur trioxide.
- That is, the balance will tip towards the item side.
- As per Le Chataliers’ standard, the overabundance pressure makes the framework yield more items
- so at last, the strain can die down by decreasing the number of particles from three to two.
- Nonetheless, a low worth of tension by and large 1 – 2 atm liked because of security and monetary worries.
The Catalyst
The impetus utilized in the contact interaction is vanadium pentoxide.
The activity of the impetus isn’t to yield more items but to make the response quicker by driving the response to arrive at balance rapidly.
In any case, the plant planned in a way to not allow the response to remain in harmony.
Clarifying the circumstances
The combination of sulfur dioxide and oxygen going into the reactor is in equivalent extents by volume.
Avogadro’s Law says that equivalent volumes of gases at a similar temperature and strain contain equivalent quantities of atoms.
That implies that the gases are going into the reactor in the proportion of 1 particle of sulfur dioxide to 1 of oxygen.
That is an abundance of oxygen comparative with the extent requested by the situation.
2SO2(g)+O2(g)⇌2SO3(g)ΔH=−196kJ/mol(6)
As indicated by Le Chatelier’s Principle, expanding the centralization of oxygen in the blend makes the place of balance shift towards the right.
Since the oxygen comes from the air, this is an extremely modest approach to expanding the transformation of sulfur dioxide into sulfur trioxide.
Temperature
Harmony contemplations:
You want to move the place of the balance quite far to one side to deliver the most extreme conceivable measure of sulfur trioxide in the balanced blend.
The forward response (the creation of sulfur trioxide) is exothermic.
2SO3(g)+O2(g)⇌2SO3(g)ΔH=−196kJ/mol(7)
As indicated by Le Chatelier’s Principle, this will be leaned toward assuming you bring down the temperature.
The framework will answer by moving the place of harmony to check this – all in all by creating more hotness.
To get however much sulfur trioxide as could reasonably be expected in the harmony blend, you want as low a temperature as could really be expected.
In any case, 400 – 450°C is definitely not a low temperature!
Rate contemplations:
The lower the temperature you use, the slower the response becomes.
A maker is attempting to create as much sulfur trioxide as could be expected each day.
It’s a horrible idea to attempt to accomplish a harmony blend that contains an exceptionally high extent of sulfur trioxide assuming it requires quite a long while for the response to arrive at that balance. You really want the gases to arrive at balance inside the extremely brief time frame that they will be in touch with the impetus in the reactor.
The split the difference: 400 – 450°C is a trade-off temperature delivering a genuinely high extent of sulfur trioxide in the balanced combination yet in an exceptionally brief time frame.
Pressure
Harmony contemplations:
2SO2(g)+O2(g)⇌2SO3(g)ΔH=−196kJ/mol(8)
As indicated by Le Châtelier’s Principle, assuming you increment the tension the framework will answer by inclining toward the response which produces fewer particles.
That will take the strain fall once more.
To get however much sulfur trioxide as could reasonably be expected in the harmony blend, you want as high a strain as could be expected.
High tensions likewise increment the pace of the response. Notwithstanding, the response is done at pressures near barometrical tension!
Financial contemplations:
Even at these moderately low tensions, there is a 99.5% chance of sulfur dioxide into sulfur trioxide. The tiny improvement that you could accomplish by expanding the tension does not merit the cost of creating those high tensions.
Impetus
Balance contemplations: The impetus has no impact at all on the place of harmony. Adding an impetus creates no more noteworthy level of sulfur trioxide in the harmony blend. Its just capacity is to accelerate the response.
Summary
The impetus utilized during the cycle is vanadium oxide.
There are three phases:
- 1 Stage: Sulfur dioxide readiness and refinement.
- 2 phase: Conversion of sulfur dioxide to sulfur trioxide by synergist oxidation.
- 3 Stage: Sulfur trioxide turns to Sulphuric corrosive.
FAQs
What are the means of the contact cycle?
Three phases.
Utilization of contact process?
In the production of sulfuric acid.
Utilization of Sulphuric corrosive?
Composts, shades, colors, drugs, explosives, cleansers, and inorganic salts and acids, as well as in petrol refining and metallurgical cycles.








