Table of Contents
Lattice Energy:
Lattice Energy is a type of potential energy that can be explained in two ways. In one definition, the lattice force is the force required to disperse solid ionic solids and convert their atomic part into gas ions. This definition causes the lattice energy value to remain constant, as this will always be an endothermic reaction. One explanation is that lattice power is a reverse process, which means that energy is released when gas ions bind to ionic solids. As stated in the description, this process will remain exothermic, and thus the lattice potential value will be negligible. Its values are usually expressed in kJ / mol units.
Lattice Energy is used to describe the stability of ionic solids. Some may expect such an ordered structure to be less stable because the entropy of the system will be lower. However, the crystalline structure allows each ion to interact with multiple opposing ions, resulting in a dramatic change in system enthalpy. More energy is released as ions charged in reverse are converted. This is what makes solid ionic substances have high melting points and boiling. Some require high temperatures to decompose before reaching the point of melting and/or boiling.
Born-Haber Cycle:
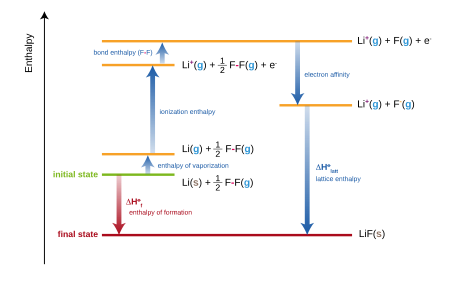
There are several important concepts to be understood before the Born-Haber Cycle is used to determine the strength of a strong ionic lattice; ionization power, electron bonding, separation power, sublimation power, heat formation, and Hess Law.
Ionisation energy is the energy needed to release an electron from a neutral atom or ion. This process always requires power input, and thus will always have a good value. Typically, ionisation forces rise from the periodic table from left to right and decrease from top to bottom. There are exceptions, usually due to the stability of the partial orbit full and complete orbitals.
Electron Affinity is the energy released when an electron is added to a neutral atom or ion. Normally, the released energy can have a negative value, but due to the definition of electron affinity, it is listed as a positive value in most tables. Therefore, when used to calculate lattice power, we must remember to remove the electron converter, not to add it. Normally, the electron affinity increases from left to right in the periodic table and decreases from top to bottom.
Dividing power is the force required to separate a compound. Composition separation is always an endothermic process, which means it will always require power input. Therefore, power conversion is always good. The magnitude of the dissociation potential depends on the electronegativity of the atoms involved.
Sublimation power is the force required to make the phase transition from solid to electric, beyond the liquid phase. This is a power input, and thus a good value. It can also be called the atomization force.
The heat of formation is the change of energy when a compound is formed from its components. This can be good or bad, depending on the atoms involved and how they interact.
Hess’s law states that a complete change of process power can be determined by breaking down the process into steps and then adding power changes to each step. The Born-Haber cycle is actually the Hess Act applied to ionic solid.
Using the Born-Haber Cycle:
The values used in the Born-Haber Cycle are all predetermined changes in the enthalpy by the processes described in the section above. Hess’s law allows us to add or subtract these values, which allows us to determine the lattice power.
- Find the strength of steel and nonmetal in their basic forms. The elements in their natural state have a zero-energy level. Remove from this heat the ionic solids that can be formed from combining these elements in the right proportions. These are strong ionic forces and will be used at the end of the process to determine the lattice strength.
- The Born-Haber Cycle requires the elements involved in turning it into their own gas forms. Add changes to the enthalpy to convert one element to its gas state, and do the same to another element.
- Metals exist naturally as individual atoms so no differentiating force needs to be added to this material. However, most nonmetals will be present as polyatomic types. For example, Cl exists as Cl2 in its original state. The energy required to convert Cl2 into Cl atoms should be added to the value obtained in 2.
- Both metal and nonmetal now need to be converted into their ionic forms, as they will be present in ionic solid. To do this, the ionisation power of the metal will be added to the value from 3. Next, the nonmetal electron coefficient will be removed from the previous value. It is released because it is the release of energy associated with the addition of electrons.
- Now metal and nonmetal will be combined to form ionic solids. This will cause the release of energy, which is called lattice energy. The lattice power value is the difference between the value from 1 and the value from 4.
FAQs
What is the difference between Hess law and the Born Haber cycle?
Hess's law states that a complete change of process power can be determined by breaking down the process into steps and then adding power changes to each step. The Born-Haber cycle is actually the Hess Act applied to ionic solid.
What is the significance of lattice power?
The lattice force provides sufficient power to drive the endothermic steps, such as the formation of cations, which are involved in the formation of the crystal lattice.
How does the Born Haber Cycle determine lattice strength?
The Born-Haber cycle is a method of analysing reaction power. The Born – Haber cycle uses Hess's law to calculate the lattice enthalpy by comparing the normal enthalpy conversion of ionic compounds from elements to the enthalpy needed to make gas ions from the elements.
How many of the following structures are involved in the Born Haber cycle?
The Born-Haber Cycle can be used to determine the strength of the ionic solid lattice; ionisation power, electron bonding, separation power, sublimation power, heat formation, and Hess Law.






