Table of Contents
Introduction
Since a chemical formula is to be expressed as a single line of chemical elements, it is generally incapable of teaching as a real structural formula, which is a metaphor for the relationship between the atoms of chemical compounds. It may also be used in chemical reactions to describe chemical reactions and other chemical reactions, such as the dissolution of ionic compounds into solutions.
Examples of Chemical Formula:
- There are 6 C atoms and 14 H atoms in a hexane molecule, which has a molecular formula of C6H14.
- The chemical formula of table salt or sodium chloride is NaCl. There is one sodium atom and one chlorine atom in each molecule.
Types of Chemical Formulas:
- There are different types of chemical formulas and each type gives us different information about the chemical. Different types of chemical formulas include: molecular, empirical, structural and abbreviated formulas.
Molecular Formula –
- Molecular Formula is also known as “true formula”, the molecular formula refers to the actual number of atoms of a molecule in a single molecule. For example, the molecular formula of sugar glucose is C6H12O6.
Empirical Formula –
- The word empirical is defined as something that is confirmed by observation. The simplest estimate of the number of items included. It’s like simplifying math fractions. Sometimes the molecular and empirical formulas are the same (e.g., H2O), and sometimes the formulas are different.
- For example, the solid formula of glucose CH2O, obtained by dividing all subscriptions to normal values (6, in this case).
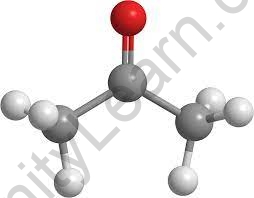
Structure Formula
- The structural formula shows both the actual number of atoms in a compound, how the atoms are arranged and which atoms are joined by each other. The structural formula indicates chemical bonds. There are different types of structure formulas, too.
- Some represent a two-dimensional structure, while others describe the arrangement of a three-dimensional atom. For example, ethanol (grain alcohol that people can drink) and dimethyl ether (a toxic compound) share similar molecular and empirical formulas.
Condensed Formula
- This type of chemical formula is a type of short note; a dense structural formula may emit carbon and hydrogen signals from a structure, simply indicating chemical bonds and functional group formulas. A written abstract formula lists the atoms in a row that appear in the cell structure.
- For example, the molecular formula of hexane is C6H14, but its condensed formula is CH3(CH2)4CH3. This formula not only provides the number and type of atoms but also indicates their position in the structure.
Hill System or Hill Notation
- Hill Notation, also known as Hill Notation, is a system for writing complex chemical formulas, molecular chemical formulas and component formulas to indicate the number of carbon atoms in a molecule first, the number of hydrogen atoms, and then their number of all other chemical elements later, in the order of the letters of the chemical symbols.
- If the formula does not contain carbon, all the elements, including hydrogen, are listed alphabetically.
- It is the most commonly used program on the chemical site and printed indexes for filtering compounds.
Examples: The following formulas are written using the Hill system, and listed in Hill order:
- BrI
- CCl4
- CH3I
- C2H5Br
- H2O4S
Rules for Writing a Chemical Formula
The following rules must be followed to compile a chemical formula for a binary combination,
- One has to know the valencies of two elements or the existing radicals.
- In the chemical formula, the total number of positive or negative valencies present in the compound should be zero. This can be done by obtaining a standard low frequency of two valencies.
- If available, you should always place the instrument at the beginning of the formula.
A chemical formula is a way of presenting information about the number of chemical atoms that make up a particular chemical or molecule, using chemical symbols, numbers, and sometimes other symbols, such as brackets, dots, brackets, commas and plus (+) and minus signals (-).
FAQ’s
What are the 3 types of chemical formulas?
There are three main types of chemical formulas: empirical, molecular and structural. Powerful formulas show the ratio of the total number of atoms in a compound, molecular formulas show the number of each type of atom in a molecule, and structural formulas show how the atoms in a molecule are connected to each other.
What is the difference between O2 and 2O?
The difference between O2 and 2O is that O2 is a molecule that contains 2 oxygen atoms while 2O contains two different oxygen atoms. The O2 molecule is stable while the 2O is ready to combine with other hydrogen-like molecules.








