Table of Contents
Introduction:
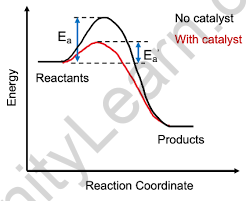
Catalysts are defined in chemistry as chemicals that change the pace of a reaction by altering the course of the reaction. A catalyst is often used to accelerate or raise the pace of a process. Catalysts, on the other hand, are employed to break or repair the chemical bonds that exist between the atoms in the molecules of certain elements or compounds. Catalysts, in essence, urge molecules to react and make the entire reaction process simpler and more efficient.
Catalysts have several key characteristics, some of which are as follows:
- A chemical reaction is not initiated by a catalyst.
- The reaction does not consume a catalyst.
- Catalysts often react with reactants to generate intermediates while also facilitating the formation of the ultimate reaction product. A catalyst can regenerate after the entire procedure.
Catalysts can be of any phase be it solid, liquid, or gaseous. Metals or their oxides, including sulfides and halides, are among the solid catalysts. Catalysts are also made from semi-metallic elements such as boron, aluminium, and silicon. Catalysts can also be liquid or gaseous materials in their pure state. These components are sometimes employed in conjunction with appropriate solvents or carriers.
A catalytic reaction is one that includes the use of a catalyst in its system. Catalytic activity, in other terms, is a chemical interaction between a catalyst and a reactant. This leads in the development of chemical intermediates, which can easily react with one another or with another reactant to generate a product.
The catalyst, on the other hand, is renewed when the reaction between the chemical intermediates and the reactants happens or takes place.
The reaction modes between the catalysts and the reactants often vary greatly, and this is especially true for solid catalysts. Acid-base reactions, oxidation-reduction processes, coordination complex creation, and free radical generation are all examples of reactions. The reaction mechanism of solid catalysts is significantly affected by surface characteristics and electrical or crystal structures.
Units
The derived SI unit for measuring a catalyst’s catalytic activity is “katal.” It is also measured in moles per second. If we were to describe a catalyst’s productivity, we might use the turnover number (or TON). The flip-over frequency (TOF), which is TON per time unit, can be used to describe the catalytic activity. Similarly, the enzyme unit is its biochemical counterpart.
Catalyst Types and Examples
Depending on the needs or requirements of the chemical process, many types of catalysts can be utilized. These are:
- Positive Catalysts
Positive catalysts are those that accelerate the pace of a chemical process. It accelerates the reaction by decreasing the activation energy barriers, allowing a high number of reaction molecules to be transformed into products, increasing the percentage of product yield.
Positive catalyst example: In the manufacture of NH3 using Haber’s method, iron oxide works as a positive catalyst, increasing ammonia output despite less nitrogen reactivity.
- Negative Catalysts
Catalysts that slow down the pace of the reaction, as well as negative catalysts It reduces the rate of reaction by increasing the activation energy barrier, which reduces the number of reactant molecules that may be converted into products and therefore the rate of reaction.
Example of a negative catalyst: Acetanilide is used as a negative catalyst to slow down the breakdown of hydrogen peroxide into water and oxygen.
- Accelerators or promoters
A promoter or accelerator is a chemical that boosts the activity of the catalyst.
In Haber’s method, for example, molybdenum or a combination of potassium and aluminum oxides operate as Promoters.
- Inhibitors or Catalyst Poisons
Catalyst poisons or inhibitors are substances that reduce catalyst action.
In the hydrogenation of an alkyne to an alkene, the catalyst palladium is poisoned with barium sulfate in quinolone solution, and the reaction is terminated at the alkene level. Lindler’s catalyst is the name given to the catalyst.
Catalysis
Catalysis occurs when a catalyst is employed to improve the pace of a chemical process.
Different Types of Catalysis
Catalysis is classified into three forms based on its nature and the physical condition of the material used in the chemical reaction.
- Homogeneous catalysis: Homogeneous catalysis refers to catalysis in which the catalyst used in the reaction and the reactants are in the same state of matter. Homogeneous catalysts operate in the same step as the reactants.
Photocatalysts
Photocatalysis is the process by which a catalyst receives light (such as visible light), gets stimulated, and then experiences an intersystem crossing with the starting material before returning to the ground state without being consumed. Following that, the excited state of the beginning material will proceed through the normal reactions. Photocatalysis, for example, is commonly used to make singlet oxygen.
- Heterogeneous catalysis: The reacting chemicals in a reaction and the catalyst used in that reaction are not in the same state of matter in this sort of catalysis.
Electrocatalysts
Multiple metal-containing catalysts are utilized in the electrochemistry context, notably in fuel cell engineering, to improve the half-reaction rates that compose the fuel cell. A typical form of the fuel cell, known as an electrocatalyst, is built on platinum nanoparticles that are supported on somewhat larger carbon particles.
- Autocatalysis: There is no particular catalyst used in the autocatalytic process. Instead, one of the products works as a catalyst, increasing the rate of product creation.
Catalyst is a term that you may come across while studying chemistry, particularly while learning about chemical processes. While some chemical reactions occur fast, others take a long time and need the use of additional materials or effort. This is where a catalyst can help.
FAQ’s
How does a positive catalyst influence the reaction?
A positive catalyst increases the pace of the reaction by modifying the route of the reaction and reducing the activation energy basis. As a result, many reactant molecules are transformed into products.
What function do promoters play in Haber's process?
Promoters and accelerators boost the activity of a catalyst in a process. Nitrogen interacts with hydrogen to generate NH3 in Haber's ammonia production method. Because nitrogen is considerably less reactive and the yield of ammonia is relatively low, NO is employed as a promoter to improve the percentage yield of ammonia produced.
What is the function of the catalyst poison in the Rosenmund reaction?
Aldehyde is produced in the Rosenmund reaction by reducing acid halides with hydrogen gas in the presence of palladium. If the catalyst is not poisoned, the reaction does not halt at the aldehyde level, which is a significant reducer of alcohol. To come to a halt at the aldehyde level. Barium sulphate is used to poison palladium.








