Table of Contents
Introduction
Hybridization occurs when two or more atoms of atoms from the same atom are mathematically assembled to produce a new orbital that is different in its components. It is just a mixture of different orbitals of the same force to form new orbitals. Hybridization was proposed as the best explanation for why all the C – H bonds in methane molecules were the same.
Hybridization State Determination:
The following are simple ways to determine if hybridization occurs or not:
You can learn more about the atom by watching it.
Instead of counting the number of bonds between atoms, count the number of connected atoms.
Count how many pairs of pairs are attached.
Add atoms and pairs alone to get the last number.
- Total 4 = Atom sp3
- Total 3 = Atom sp2
- Total 2 = Atom sp
Apart from the above methods, there is another way to determine the state of hybridization. However, the second method may not always be accurate.
Formula:
0.5 (V + M-C + A)
V = Total number of valence electrons in the middle atom
M = number of Monovalent atoms
C = Cation charge total
A = Anion charge amount
SF6 (Sulphur Hexafluoride) – Symptoms, Hybridization, and Geometry:
Sulphur hexafluoride is a greenhouse gas, non-flammable and colourless and odourless. Inorganic and non-polar. SF6 is usually made by producing or combining S8 and F2.
SF6 Features:
- Chemically, SF6 gas is extremely stable.
- It is neither hot nor high in power, it has a dielectric surface about 2.5 times that of air.
- It is used as a power supply, power supply and cooling system for switchgear, transformers and substations. nonpolar organic solvents but not in water.
- In the air, sulphur hexafluoride is a concentrated gas that usually stays low.
Hybridization and Geometry SF6:
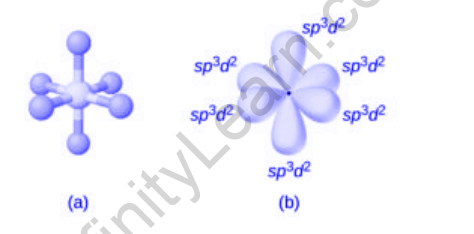
SF6 hybridization is sp3d2. Let’s see how? Orbitals involved in the synthesis of sulfur hexafluoride, as well as bonds formed during the interaction of sulfur and fluorine molecules, decided to combine.
However, when it shares electrons and is excited, the electron pairs in both 3 and 3p orbitals are paired. These electrons fly high to fill the top 3d empty orbitals. Then six hybrid orbital structures were formed:
- another 3s
- three of 3p
- two 3d
All six additional partial orbitals get mixed up now. This results in the production of six sp3d2 hybrid orbitals. The sp3d2 hybrid orbitals also overlap with the 2p fluorine orbital, forming an S-F bond.
SF6 Geometry:
With Sulphur Hexafluoride, the main element Sulphur and fluorine atoms are evenly distributed in it. Atoms are arranged in an octahedral arrangement, resulting in an octahedral molecular
Name Of molecule: Sulphur hexafluoride molecular
formula: SF6
hybridization Type: sp3d2
bond angle: 90o
geometry: Octahedral
What is the Hybridization of Sulphur Hexafluoride?
To determine the mixing of sulphur hexafluoride we will look at the orbitals involved and the bonds formed during the fusion of the sulphur and fluorine molecules. The orbitals involved are 3s, 3py, 3py, 3pz and 3dx2 – y2 and 3dz2. During the formation of SF6, the central atom at its base will have a 3s23p4 configuration. In the fun mode, the electron pairs at 3 and 3px orbitals are not matched and one pair per pair is upgraded to 3dz2 and empty 3dx2-y2. Now all these six orbitals (one 3s, three 3p and two 3d) full half get a mix that leads to the formation of six sp3d2
hybrid orbitals. In addition, the sp3d2 hybrid orbitals overlap with the 2p orbital fluorine, and form an S-F bond.
Important Points to Remember
- There are six mixed orbital structures.
- One 3s-orbital, 3p-orbital and two 3d-orbital participate in the merger.
- Six sp3d2 hybrid orbitals are measured at six angles of a normal octahedron.
SF6 molecular geometry will be octahedral because if we look at the structure the sulphur hexafluoride has a central sulphur atom where 12 electrons or 6 pairs of electrons are present and there are no single pairs. F-S-F bonds are set at 90 degrees.
Types of Hybridization
Hybridizations vary in nature and the number of atomic orbitals involved. Below are its types:
- sp hybridization
- sp2 hybridization
- sp3 integration
- sp3d hybridization
- sp3d2 hybridization
- sp3d3 hybridization
FAQs
What Are the Signs of SF6 Gas?
SF6 is colourless, odourless, non-flammable, and non-toxic. It probably does not work, which means it is stable and does not work with other chemicals under normal conditions.
Is SF6 Gas Harmful to the Environment?
This gas does not deplete the ozone layer or cause atmospheric pollution. However, it is 24,000 times more efficient than carbon dioxide (CO2) in the heat trap, making SF6 a more powerful greenhouse gas. This is why it is so important to keep an eye on the SF6 level in the gears to change leaks.
Q: Write a Few Features of Hybridization?
Ans: A few aspects of hybridization are as follows.
1. Hybridization is a mixture of orbitals and not electrons. So in a full, half-empty and empty combination, it may play a role.
2. The number of compound orbital structures is proportional to the number of rotating atoms that may be involved in the fusion process.
3. Each hybrid orbital has two lobes, one long and the other small. The bond will be formed from a large lobe.
4. Number of orbitals that are a mixture of atoms in the middle of a molecule or ion = number of bonds sigma + one pair of electrons.
Q: What makes SF6 gas such an excellent dielectric medium?
Ans: Dielectric refers to the ability to carry electricity without the presence of conductivity. Dielectric power is related to the fact that something can withstand high electrical energy without damage.
The ability to receive free electrons is called electronegativity, and fluorine is the most powerful electronegative factor known to exist. SF6 is made up of six fluorine atoms that can be separated from sulfur atoms. It captures electrons during arc and then returns to its original position.
Its strong dielectric power and electronegativity make it a natural gas to protect T&D energy assets.
Q: What are the key features and types of Hybridization?
Ans: Key features are as follows:
- The number of initial orbital bonds is equal to the number of hybrid orbitals produced.
- Pure space is not in mixed orbitals.
- Hybrid orbitals are similar in shape and strength
- Hybridization can occur in both partially or fully filled orbitals.
- The energy levels of the orbitals involved in the merger should be the same or similar.