Table of Contents
What are Carbon Allotropes?
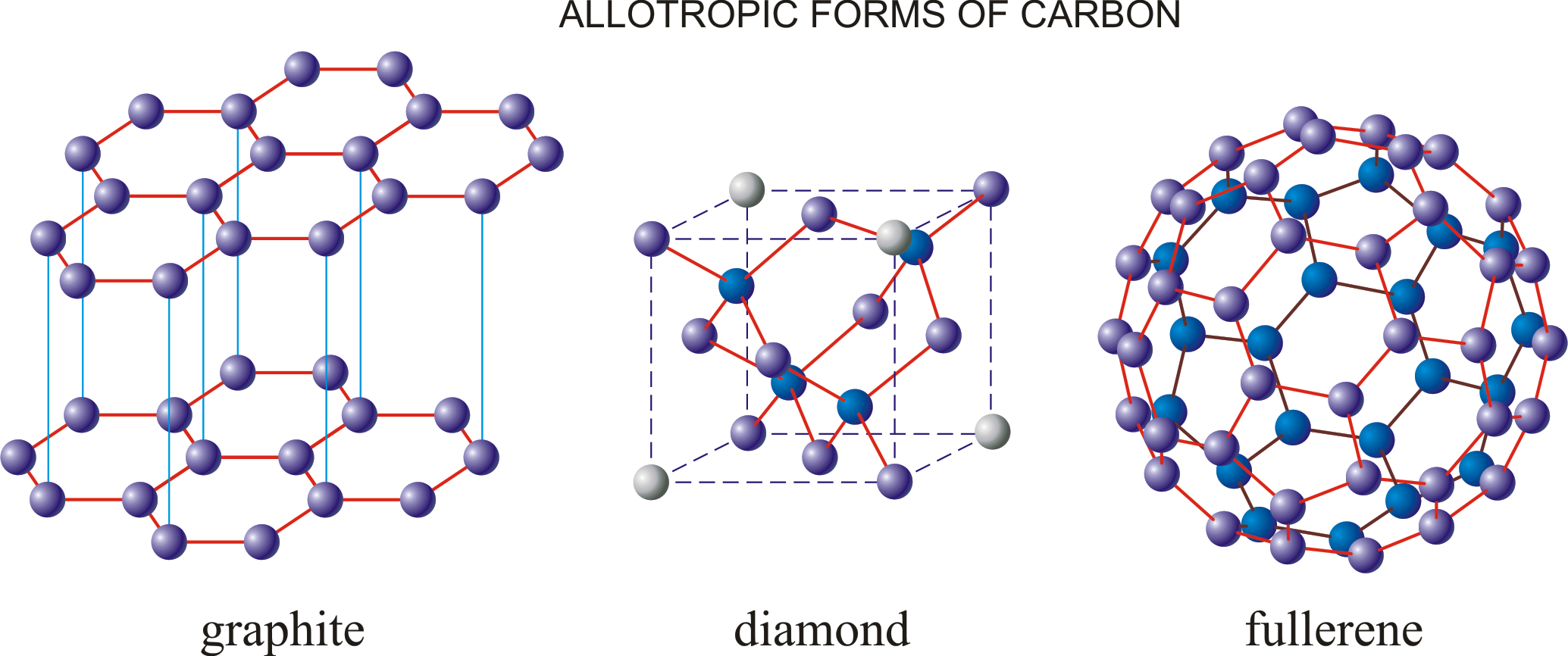
Carbon, with an atomic number of 6, and a symbol of ‘C’ in the periodic table, is one of the most powerful elements we encounter. Carbon is an example of an element that exhibits allotropy. Carbon allotropes can be amorphous or crystalline in nature (Diamond, Graphite).
Carbon is one of the few elements that can have different allotropic forms due to its capacity to have variable oxidation states or coordination numbers. Another important component is carbon’s capacity to catenate. As a result, it leads to the development of diverse carbon allotropes.
What is the total number of Carbon Allotropes?
- Diamond is a clear, highly hard crystal with carbon atoms organised in a tetrahedral lattice. This carbon allotrope has a low electrical conductivity but a high heat conductivity.
- Lonsdaleite is also known as hexagonal diamond.
- Other allotropes, nanotubes, charcoal, and fullerenes all contain graphene as a structural constituent.
- Q-carbon: These ferromagnetic carbon allotropes have a strong, dazzling crystal structure that is harder and brighter than diamonds.
- Graphite is a soft, black, flaky material that is a moderate conductor of electricity. The carbon atoms are linked together in flat
- hexagonal lattices (graphene), which are subsequently stacked into sheets.
- Carbon acetylenic linear (Carbyne)
- Carbon that is amorphous
- Buckminsterfullerene, popularly known as “buckyballs,” is a kind of fullerene that includes C60.
- Carbon nanotubes are carbon allotropes with a small diameter.
Graphite
- It’s also a completely pure type of carbon. This carbon allotrope is made up of hexagonally organised flat two-dimensional layers of carbon atoms. It’s a supple, dark, and slick solid. Graphite retains this feature because it cleaves easily between layers.
- Each C atom in each layer is connected to three other C atoms by a C-C covalent connection. Every carbon in this image is sp2 hybridised. A pi bond is generated as the fourth bond. The -electrons are mobile and can carry electricity because they are delocalized.
- There are two types of graphite: ß and ß.
- The layers are stacked in ABAB order in the form, with the third layer directly above the first.
- The layers in the ß form are ordered ABCABC.
Graphite’s properties:
- Include the ability to function as a lubricant since the layers are layered on top of each other.
- It also has a metallic shine, which aids in electrical conductivity. It is an excellent heat and electrical conductor.
One of the graphite’s most important qualities is that it may be used as a dry lubricant in high-temperature devices where oil cannot be used. - Graphite is used to manufacture crucibles with the feature of being inert to both dilute acids and alkalis.
Structure of Carbon Allotrope (Graphite):
Graphite has a honeycomb layered structure, which is unique among carbon allotropes. Each layer is made up of carbon atoms arranged in planar hexagonal rings with a carbon-carbon bond length of 141.5 picometers.
Three of the four carbon atoms create sigma bonds, while the fourth forms a pi bond. Vander Waal forces bind the layers of graphite together.
Other Carbon Allotropes
- Buckminsterfullerene (C60) is another type of carbon allotrope. Fullerene has a cage-like structure that gives it the appearance of a football.
- Fullerenes
They are spheroidal molecules with the formula C2n, where n is less than 30. These carbon allotropes can be made by laser evaporating graphite. - Fullerenes, unlike diamonds, dissolve in organic solvents. Buckminster Fullerene is the name given to the fullerene C60. The carbon atoms have undergone sp2 hybridization.
- Buckminsterfullerene, or simply fullerene, is a kind of fullerene. Fullerene is a 60-vertices saucer-ball molecule with a carbon atom at each vertex. It consists of 20 six-member rings and 12 five-member rings.
- Five-membered rings can only be fused to other five-membered rings, but six-membered rings can only be fused to other six-membered rings. There are single and double carbon-carbon bonds with lengths of 142pm and 138.3pm, respectively.
Fullerene’s Characteristics
- The behaviour and structure of fullerene alter as the temperature varies. At a higher temperature, the fullerene is converted to the C70 form.
- The structure of fullerene varies as pressure changes.
- The ionisation enthalpy of fullerene is 7.61 electron volts.
- The electron affinity of fullerene is 2.6 to 2.8 electron volts.
- Fullerene (C60) acts as an electrophile in chemical reactions.
- Fullerene can accept electrons and act as an electron acceptor. It is easily capable of receiving three or more electrons. As a result, it has the ability to serve as an oxidizer.
- Fullerenes are doped with alkali or alkaline earth metals to achieve superconductivity.
- Ferromagnetism is a characteristic of fullerene.
Uses of Fullerene
- Fullerene-based conductors are used.
- It is capable of absorbing gases.
- Fullerene-based lubricants are used.
- Fullerenes are used in the production of a variety of cosmetics-related goods.
- Carbon nanotubes are made out of graphene sheets.
- In various methods, fullerenes are used in biological applications.
Silicates
- Silicates are formed by fusing alkali oxides with SiO2. There are discrete tetrahedral units in them. Silicon has been hybridised with sp3. The structures of these carbon allotropes are used to classify them.
1. Orthosilicates: Orthosilicates are made up of distinct SiO4 units. Willemite, for example (ZrSiO4).
2. Pyrosilicate: A bond between two units is formed by an oxygen atom. Si2O76- is the simplest ion of this kind. Thortveite, for example (Sc2[Si2O7]).
3. Cyclic Silicates: Two oxygen atoms are shared by the units. Only two ions, Si3O96- and Si6O1812-, are currently known. Beryl, for example, is Be3Al2Si6O18.
4. Chain Silicates: Chain silicates are formed when the units are linked in a linear fashion. There are two kinds of them:
FAQs
What is Carbon's purest form?
Fullerene is the purest form of carbon because it lacks the gleaming edges and surface bonds that attract other atoms found in graphite and diamond.
How does fullerene come to be?
The condensation of Cn small molecules produces a sooty substance when graphite is heated in an electric arc in an inert atmosphere such as helium or argon, culminating in fullerene. The C60 and C70 fullerenes contained in sooty material are separated from the fullerenes found in soot by extraction with benzene or toluene followed by chromatography over alumina.