Table of Contents
Introduction
National Testing Agency (NTA) conducts the JEE (joint placement test) consistently. It is a stage to give admissions to the hopeful competitors the nation over, into the lofty specialized schools of our country.
Competitors should work vigorously with escalated difficult work and concentration to acquire progress in this assessment.
The inquiries posed in science are a lot more straightforward and take lesser time when contrasted with the inquiries posed in physical science or math. A couple of significant sections and a few ideas are profoundly critical as there is an example wherein these inquiries rehash consistently.
Going through these inquiries will give you a fair thought regarding your readiness status and assist you with using the excess time before the assessment.
Significant Question For States Of Matter
What are the absolute most significant subjects in conditions of issue?
ANS: States of the issue falls under the significant subjects, which help you in scoring great in JEE. Following are the ideas that ought to be concentrated cautiously:
- Various conditions of issue fluid, gases, solids
- Van Der Waals powers
- Properties of gases and gas regulations
- Dynamic Theory of Gases
- Presumptions made for Kinetic Theory of Gases
- Optimal Gas Law and Dalton’s Law of Partial Pressure
- Graham’s law of dissemination and Gas audiometry
- Deviation from ideal gas conduct and Van Der Waal’s Equation
Territories of Matter Previous Year Questions with Solutions are given here. The three essential conditions of the issue are strong, fluid and gas. The matter exists in nature in various structures. A few substances are inflexible and have a decent shape like stone and wood. A few different substances can stream and take the state of their compartment like water, while some don’t have unequivocal shape or size, like air.
Significant subjects in conditions of issue incorporate, various conditions of issue fluid, gases, solids, Van Der Waals powers, Properties of gases and gas regulations, Kinetic Theory of Gases, Assumptions made for Kinetic Theory of Gases, Ideal Gas Law and Dalton’s Law of Partial Pressure, Graham’s law of dissemination and Gas Eudiometry, Deviation from ideal gas conduct and Van Der Waal’s Equation.
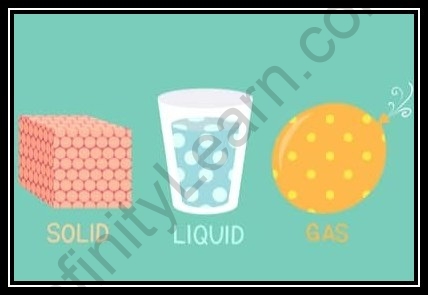
States of Matter
states of matter are of prime significance to physicists. Regular components and mixtures structure three actual conditions of issue, but there are numerous different states, more uncommon however similarly significant.
The fluid gem condition of specific mixtures has the properties of solids as well as fluids and is the premise of electronic presentations. More states are gotten when the particles are lighter.
The electrons in metals and earthenware production go through a change because of which power is led without dissemination.
- In this manner, the matter is ordered essentially into three classifications relying on its actual state specifically strong, fluid and vaporous states.
Three conditions of issue strong fluid and gas
- Solids have a distinct volume and shape: Definite shape and volume of the solids is a consequence of solid powers of fascination between its constituent particles which hold them together in a proper position and game plan.
- Fluids likewise have an unmistakable volume however no unequivocal volume: They come to fruition of the compartment in which they are set. This is because of the somewhat more fragile power of fascination among the particles of the fluid.
- Gases have neither a positive volume nor a distinct shape: They fill the compartment totally in which they are set. This is because of the way that the power of fascination among the particles of fluids is just about nothing and they are allowed to move autonomously to one another.
How to Approach an inquiry in conditions of issue?
Issue: A gas possesses one litre under barometrical tension. What will be the volume of a similar measure of gas under a tension of 750 mm of Hg at a similar temperature?
Arrangement:
Given
V1 = 1 liter
P1 = 1 atm =760 mm of Hg
V2 =?
P2 = 750 mm of Hg
Utilizing P1 V1 = P2V2
We get
760 × 1 = 750 × V2
V2 = 1.0133 liter = 1013.3 ml
FAQs
Q: How might I read up for the JEE Main States of Matter?
Ans: To read up for the JEE Main ‘Provinces of Matter’, first, make a reasonable report plan for yourself. Plan your timetable so that you get familiar with the material for the test more than half a month to a couple of months before the test date. This will give you sufficient opportunity to study without surging. Likewise, try to differ your review plan; read from the coursebook one day, then, at that point, tackle past papers the following, then, at that point, watch a video exposition of the subject the following day. This will separate the repetitiveness of examining and allow you to learn in a pleasant manner.
Q: How might I utilize JEE Main ‘Provinces of Matter’ Important Questions while examining?
Ans: There are a couple of manners by which you can utilize the JEE Main ‘Provinces of Matter’ Important Questions while examining. To start with, you can involve it as an aide so you know which subtopics in the section are probably going to show up in the test. You can concentrate your investigation of this section on those subtopics specifically, while additionally going over the subtopics that aren’t canvassed in the States of Matter Important Questions. We propose that you endeavour to address the inquiries on your own first, without checking the responses out.
Whenever you have settled the inquiries, go through the responses we have given so you can see how you were correct and how you veered off-track. This will permit you to acknowledge which regions you really want to focus on. You can then revise your review timetable to zero in on those areas.
Q: What does ‘States of Matter’ Mean?
Ans: To lay it out plainly, the ‘Territories of Matter’ alludes to the three fundamental “shapes” that all matter in the realized universe takes: Solid, Liquid, or Gas. These structures rely upon the thickness of the atoms making up the substance being referred to. Assuming a substance has thickly loaded atoms with practically zero space between them, the substance will no doubt be a Solid. Assuming that the atoms of a substance are all the more approximately pressed together, with some space between them, the substance is probably going to be a Liquid. At long last, assuming the particles of a substance have a lot of room in the middle of them, then, at that point, the substance is possible a Gas.