Table of Contents
Introduction:
To lay it out plainly, the ‘States of Matter’ alludes to the three fundamental “frames” that all matter in the realized universe takes: Solid, Liquid, or Gas. These structures rely upon the thickness of the particles making up the substance being referred to. Assuming that a substance has thickly loaded particles with practically zero space between them, the substance will no doubt be a Solid. On the off chance that the atoms of a substance are all the more inexactly pressed together, with some space between them, the substance is probably going to be a Liquid. At last, assuming that the particles of a substance have a lot of room in the middle of them, then, at that point, the substance is probable a Gas.
Previous Year Solved Questions of States Of Matter (JEE Main)
Question: Assuming that Z is the compressibility factor, van der Waals’ condition at low strain can be composed as :
(1) Z=1-Pb/RT
(2) Z=1+Pb/RT
(3) Z = 1+RT/Pb
(4) Z = 1-a/VmRT
Answer:
(P+a/Vm2 )(Vm-b) = RT [Van der waals condition of state]
Vm-b ≈ Vm
So the condition becomes (P+a/Vm2 )Vm= RT
⇒PVm+a/Vm = RT
Partition all terms by RT
PVm/RT+a/VmRT = RT/RT
PVm/RT = 1-a/VmRT
Z = 1-a/VmRT [Z = PVm/RT]
Consequently choice (4) is the response.
Question: Which intermolecular power is generally mindful in permitting xenon gas to condense?
(1) Instantaneous dipole instigated dipole
(2) Ion dipole
(3) Ionic
(4) Dipole-dipole
Answer:
For the liquefaction of xenon, prompt dipole initiated dipole powers are mindful.
Thus choice (1) is the response.
Question: The temperature at which oxygen particles have a similar root mean square
speed, as helium molecules have at 300 K, is :
(Nuclear masses : He = 4 u, O = 16 u)
(1) 1200 K
(2) 600 K
(3) 300 K
(4) 2400 K
Answer:
Given Atomic masses : He = 4 u, O = 16 u
(Vrms) O2 = (Vrms) He
√(3RT1/M1) = √(3RT2/M2)
T1/M1 = T2/M2
T1/32 = 300/4
T1= 300×32/4
= 2400 K
Subsequently choice (4) is the response.
Question: The compressibility factor for a genuine gas at high tension is :
(1) 1-Pb/RT
(2) 1+ RT/Pb
(3) 1
(4) 1+Pb/RT
Answer:
(P+a/V2)(V-b) = RT [Real gas equation]
a/V2 can be dismissed at high tension.
PV-Pb = RT
PV/RT = (RT/RT) + (Pb/RT)
PV/RT = 1 + (Pb/RT) … (1)
Z = PV/RT … (2)
Likening (1) and (2)
Z = 1 + (Pb/RT)
Thus choice (4) is the response.
Question: The relationship among most likely speed, normal speed and root mean
Square speed is individual:
(1) √2 : √(8/π) : √3
(2) √2 :√3 : √(8/π )
(3) √3 : √(8/π): √2
(4) √(8/π) : √3: √2
Answer:
Vmpv = √(2RT/M)
Vav = √(8RT/πM)
Vrms = √(3RT/M)
Vmpv : Vav : Vrms . = √(2RT/M) : √(8RT/πM) : √(3RT/M)
= .√2 : √(8/π) : √3
Thus choice (1) is the response.
Question: Worth of gas consistent R is
(1) 0.082 L atm
(2) 0.987 cal mol-1 K-1
(3) 8.3 J mol-1 K-1
(4) 83 erg mol-1 K-1
Answer:
R = 8.3 J mol-1 K-1
Henceforth choice (3) is the response.
Question: By the number of folds the temperature of a gas would increment when the root mean
Square speed of the gas particles in a holder of fixed volume is expanded from 5×104cm/s to 10×104 cm/s?
(1) Four
(2) three
(3) Two
(4) Six
Answer:
Vrms ∝ √T
V1/V2 = √(T1/T2) = 5×104/10×104
figuring out, we get
T1/T2 = 25/100 = ¼
T2 = 4T1
Henceforth choice (1) is the response.
Question: Active hypothesis of gases demonstrates
(1) Only Boyle’s regulation
(2) Only Charle’s regulation
(3) Only Avogadro’s regulation
(4) these
Answer:
One of the proposes of the dynamic hypothesis of gases is normal active energy corresponding to T.
This hypothesis demonstrates all the above-given regulations.
Subsequently choice (4) is the response.
Question: Which one of coming up next is some unacceptable presumption of the active hypothesis of gases?
(1) All the atoms move in an orderly fashion among impact and with a similar speed.
(2) Molecules are isolated by significant stretches contrasted with their sizes.
(3) Pressure is the consequence of the flexible crash of particles with the holder’s divider.
(4) Momentum and energy generally stay monitored.
Answer:
The particles are generally in arbitrary movement and submit to Newton’s law of motion. They have speeds every which way going from zero to limitlessness.
Henceforth choice (1) is the response.
Question:
‘a’ and ‘b’ are Vander Waal’s constants for gases. Chlorine is more handily liquified than ethane on the grounds that:
(1) a for Cl2 < a for C2H6 but b for Cl2 > b for C2H6
(2) a for Cl2 > a for C2H6 but b for Cl2 < b for C2H6
(3) an and b for Cl2 > an and b for C2H6
(4) an and b for Cl2 < an and b for C2H6
Answer:
More prominent the ‘a esteem, all the more effectively the gas is liquified, bring down the ‘b’ esteem, all the more effectively the gas is liquified.
Thus choice (2) is the response.
Question: A vaporous compound of nitrogen and hydrogen contains 12.5% (by mass) of
Hydrogen. The thickness of the compound compared with hydrogen is 16. The sub-atomic recipe of the compound is :
(1) NH2
(2) NH3
(3) N3H
(4) N2H4
Answer:
Considering that vaporous compound of nitrogen and hydrogen contains 12.5% (by mass) of
Hydrogen.
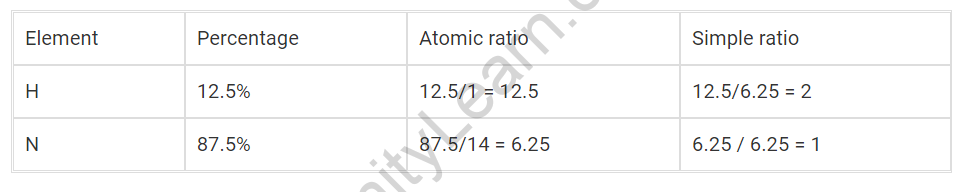
Observational recipe = NH2
Observational mass = 16
Sub-atomic weight = 2× Vapor thickness = 2×16 = 32
So n = sub-atomic mass/experimental mass = 32/16 = 2
Sub-atomic recipe = Empirical equation × n
= (NH2)×2
= N2H4
Subsequently choice (4) is the response
Question: The one that is widely utilized as a piezoelectric material is
(1) quartz
(2) undefined silica
(3) tridymite
(4) mica
Answer:
Quartz is utilized as a piezoelectric material.
Henceforth choice (1) is the response.
Question: Which crude unit cell has inconsistent edge lengths and all hub lengths not quite the same as 900.
(1) Monoclinic
(2) Triclinic
(3) Tetragonal
(4) Hexagonal
Answer:
The triclinic crude unit cell has inconsistent edge lengths and all pivotal lengths not the same as 900.
Subsequently choice (2) is the response.
Question: In Van der Waals condition of the gas regulation, the steady b is a proportion of
(1) Intermolecular shocks
(2) Intermolecular fascination
(3) Volume involved by atoms
(4) Intermolecular crashes per unit volume.
Answer:
Van der Waals steady b is the proportion of viable volume involved by the gas atoms.
Subsequently choice (3) is the response.
Question: A tension cooker lessens preparing time for food in light of the fact that
(1) Heat is all the more equally dispersed in the cooking space.
(2) B.P of the water engaged with cooking is expanded
(3) The higher tension inside the cooker squashes the food.
(4) Cooking includes substance changes helped by an increase in temperature.
Answer:
By Gay Lussac’s regulation, at steady tension of a given mass of a gas is straightforwardly corresponding to the outright temperature of the gas. So on expanding pressure, temperature additionally increments. So the edge of boiling over water is likewise expanded.
Consequently choice (2) is the response.
Question: As per the motor hypothesis of gases, in an optimal gas, between two progressive impacts a gas particle ventures
(1) In a roundabout way
(2) In a wavy way
(3) In an orderly fashion way
(4) with a sped up speed
Answer:
As indicated by the motor hypothesis of gases, in an optimal gas, between two progressive impacts a gas atom goes in an orderly fashion way.
Subsequently choice (3) is the response.
FAQs
What number of conditions of issue are there in science?
The response is that there are four basic conditions of issue - strong, fluid, gas and plasma. These are the ones that happen normally in the Universe.
For what reason do conditions of the issue have various properties?
There are three conditions of issue: strong; fluid and gas. They have various properties, which can be made sense of by checking out at the game plan of their particles. This is the hypothetical temperature at which particles have minimal measure of energy and the slowest development.
Which condition of issue can be packed or crushed without any problem?
Gases, The particles and atoms in gases are considerably more fanned out than in solids or fluids. They vibrate and move unreservedly at high velocities. A gas will fill any holder, yet on the off chance that the compartment isn't fixed, the gas will get away. Gas can be packed substantially more effectively than a fluid or strong.







