Table of Contents
Free radicals are a group of damaged electrons found in practically every molecule that swiftly react with other ingredients and produce the desired consequences. Many organic molecules have a short life, which is why even free radicals must multiply from time to time to prevent being completely destroyed by a single body.
These free radicals are essential for the healthy functioning of the body, which is why there should be more of them in humans’ bodies.
There are several examples of free radicals in chemistry, including Nitric Acid (NO), Hydrogen Peroxide, Hydroxyl radical (OH), and others.
A free radical is a molecule type that may hold an unpaired electron in its atomic orbital and live independently. Because of the unpaired electron, all radicals have several traits in common.
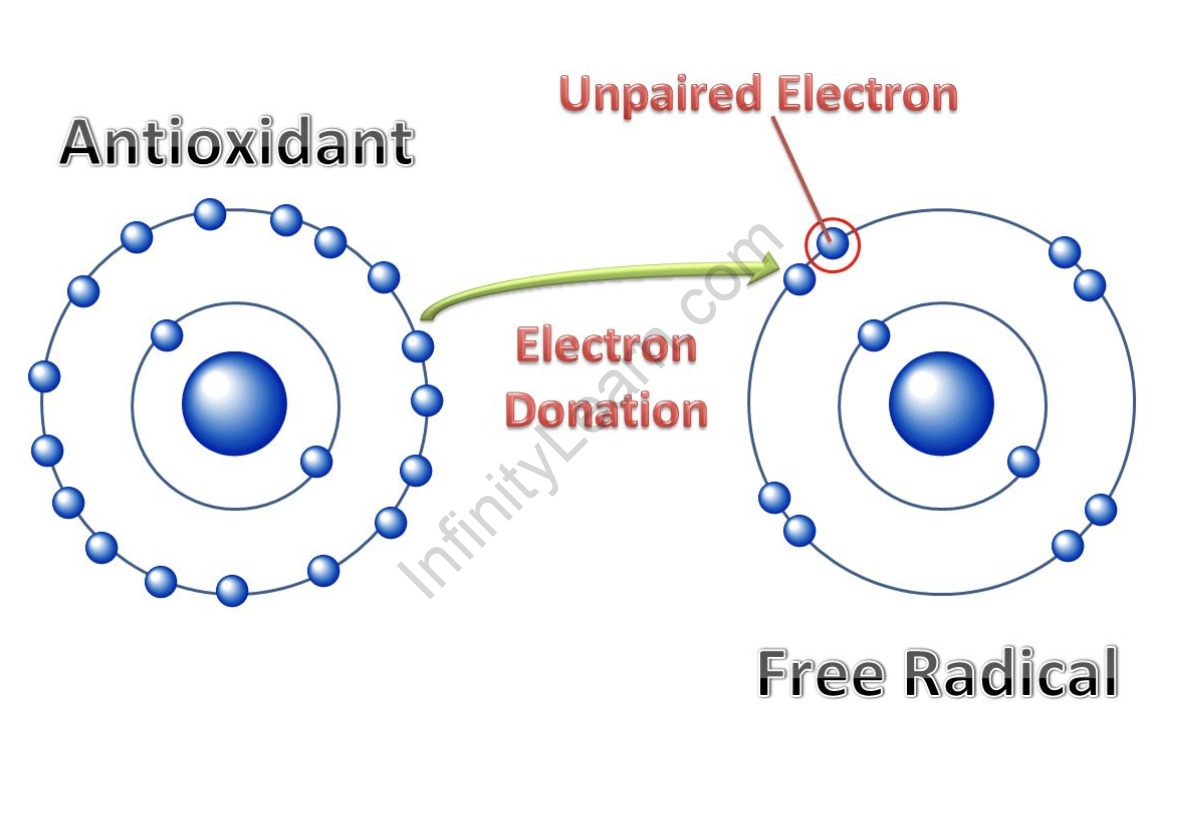 Molecules often have bonding electron pairs and lone pairs, which are non-bonding electron pairs or un-shared electron pairs. Based on Pauli’s exclusion principle, each bonding or nonbonding electron pair contains two electrons in opposing spin orientation, +1/2 and -1/2 in one orbital, whereas an unpaired electron is a single electron alone in one orbital. A molecule with an unpaired electron is referred to as a free radical, and it is a paramagnetic species.
Molecules often have bonding electron pairs and lone pairs, which are non-bonding electron pairs or un-shared electron pairs. Based on Pauli’s exclusion principle, each bonding or nonbonding electron pair contains two electrons in opposing spin orientation, +1/2 and -1/2 in one orbital, whereas an unpaired electron is a single electron alone in one orbital. A molecule with an unpaired electron is referred to as a free radical, and it is a paramagnetic species.
Free Radical Properties
- Free radicals are a distinct and uncommon species that exist only under very specific and constrained circumstances. Some free radicals, on the other hand, are all too known to us.
- Molecular oxygen is an example of a free radical and a biradical species. According to Hund’s rule, standard and stable molecular oxygen is in a triplet state, and the two unpaired electrons have the same spin orientation in two orbitals and so have the same orbital energy.
- Nitrogen monoxide and nitrogen dioxide are also free radical species that are stable. Furthermore, oxygen-free radicals, such as superoxide anion radicals and singlet molecular oxygen, are implicated in immunity.
- As a result, free radicals are quite known to us in our daily lives and are extremely significant substances.
- Free radicals are extremely reactive and unstable. They may donate or take electrons from other molecules, allowing them to operate as oxidants or reactants.
- The hydroxyl radical is the most significant oxygen-containing free radical.
- The radical superoxide anion
- Peroxide of hydrogen
- Hypochlorite
- The radical nitric oxide
- The radical peroxynitrite
Different Kinds of Free Radicals
Free radicals are classified into two categories. They are as follows:
- Neutral Free Radicals
Because these free radicals are not charged, they have substantially lower reactivity than charged free radicals.
- Free Radicals With Charges
The charged free radicals are extremely reactive and have a high ability to combine with the other components and constituents. These free radicals can be either positive or negative in nature.
Furthermore, there are two kinds of radicals: sigma radicals and pi radicals. Unpaired electrons in the sigma-radical are in the sigma orbital, whereas unpaired electrons in the pi radical are in the pi orbital. As a result, the radicals mentioned above are pi radicals, and the t-Butyl radical is likewise a pi radical since it is stabilized by hyperconjugation. The phenyl radical and the vinyl radical, on the other hand, are typical sigma radicals.
Normally, the hyperconjugation or resonance effects stabilize pi radicals. However, because there is no such stabilizing effect, sigma radicals are extremely reactive.
What Gives Rise to Free Radicals?
Free radicals can be produced by metabolic activities that appear to be anticipated in the body. These metabolic activities are important and required by the body, which is why absolute eradication is unattainable and hazardous for the organism.
External sources of free radicals, such as X-rays and cigarette smoke, can potentially enter the body.
Free radicals in living beings’ bodies induce rapid combinations with other components and, as a result, build enormous reaction chains. These radicals react quickly with every molecule, and these reactions are known as ‘oxidation reactions’ in living creatures, including humans.
Free Radical Uses
- These extremely reactive structures are found in the membranes of cells that contain potentially harmful physiologically relevant substances such as DNA, lipids, proteins, and carbohydrates, among others.
- Free radicals target essential macromolecules, causing cell damage and disrupting homeostases, such as proteins and nucleic acids.
- In general, alkyl or aryl halides are utilized as radical precursors for R or Ar, although halogenation of sugars and nucleosides with numerous OH groups and other sensitive functional groups is challenging.
- The Barton Mccombie reaction is extremely beneficial in radical reactions involving sugars, nucleosides, and peptides.
- In addition to methyl xanthate, other thiocarbonyl derivatives produced from alcohols using phenoxythiocarbonyl chloride, diimidazole, and others can be employed.
Free Radicals’ Origins
Internally, free radicals are produced through the following mechanisms.
- Mitochondria
- Inflammation
- Exercise
- Phagocytosis
- Peroxisomes
Externally, free radicals can be found in the following places.
- Pollution of the environment
- Tobacco smoke
- Pesticides and radiation drugs
- The ozone layer
FAQ’s
Q. What causes free radicals to be so reactive?
ANS: Radicals are extremely reactive, requiring a great deal of energy to form. When discussing radical reactivity, “more reactive” often refers to a step toward higher exothermic abstraction of hydrogen atoms. As a result, the reaction is less susceptible to the stability of the carbon-centric radical.
Q. What are the effects of free radicals on the body?
ANS: In the body, free radicals produce comparable deterioration by killing cell membranes and making cells prone to decline and diseases. These free radicals wreak havoc on DNA and mitochondria, the essential building blocks of all tissues, and cause a slew of health concerns in their wake.
Q. In chemistry, what is a radical?
ANS: A radical is a chemical substance that has an unpaired electron. A radical might be electrically neutral, positively charged radical cation, or negatively charged radical anion. Examples of Radicals; Under UV light, the chlorine molecule Cl2 undergoes hemolysis, resulting in the formation of two Cl radicals.
Q. What illnesses are caused by free radicals?
ANS: There is mounting evidence that the majority of the degenerative illnesses that affect humanity are caused by harmful free radical responses. Atherosclerosis, cancer, inflammatory joint disease, asthma, diabetes, senile dementia, and eye degeneration are examples of such diseases.





