Table of Contents
The theory of valence shell electron pair repulsion (VSEPR) predicts the geometry of individual molecules based on the number of electron pairs surrounding their central atoms. Ronald Gillespie and Ronald Nyholm, the theory’s two major developers, are also named after it. The premise of VSEPR is that the valence electron pairs surrounding an atom repel each other and, as a result, will arrange themselves in a way that minimises this repulsion.
This, in turn, reduces the energy of the molecule and increases its stability, determining the molecular geometry. Gillespie has emphasised that the electron-electron repulsion caused by the Pauli exclusion principle is more important than electrostatic repulsion in determining molecular geometry. The insights of the VSEPR theory are derived from a topological analysis of a molecule’s electron density. The electron localization function (ELF) and the quantum theory of atoms in molecules are two examples of quantum chemical topology (QCT) methods (AIM or QTAIM).
As a result, VSEPR has nothing to do with wave function-based methods like orbital hybridisation in valence bond theory. VSEPR theory is used to predict how electron pairs will be arranged around central atoms in molecules, particularly simple and symmetric molecules. In this theory, a central atom is one that is bonded to two or more other atoms, whereas a terminal atom is bonded to only one other atom.
The two carbons and one nitrogen, for example, are central atoms in the molecule methyl isocyanate (H3C-N=C=O), while the three hydrogens and one oxygen are terminal atoms. After drawing the Lewis structure of the molecule and expanding it to show all bonding groups and lone pairs of electrons, the number of electron pairs in the valence shell of a central atom is determined. In VSEPR theory, a double or triple bond is treated as a single bonding group.
The steric number of a central atom is the sum of the number of atoms bonded to it and the number of lone pairs formed by its nonbonding valence electrons. The geometry of the central atoms and their non-bonding electron pairs determines the overall geometry of the molecule.
Overview
The VSEPR theory is used to predict the shape of molecules based on the electron pairs that surround the molecule’s central atoms. Sidgwick and Powell proposed the theory for the first time in 1940. The VSEPR theory is predicated on the assumption that the molecule will take a shape that minimises electronic repulsion in the valence shell of that atom. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion with a nonmetal central atom, as well as the structure of many molecules and polyatomic ions with a metal central atom. The premise of the VSEPR theory is that electron pairs in bonds and lone pairs repel each other and, as a result, will adopt a geometry that places electron pairs as far apart as possible. This theory is overly simplistic and ignores the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds in a way that the Lewis electron-pair approach does not.
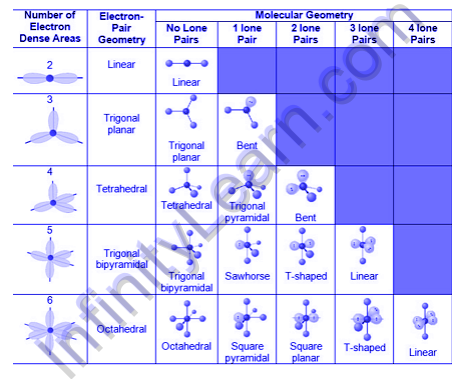
The molecule or polyatomic ion is designated as AXmEn in the VSEPR model, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group (typically a lone pair of electrons), and m and n are integers. Each group that wraps around the central atom is referred to as a bonding pair (BP) or a lone (nonbonding) pair (LP). We can predict the relative positions of the atoms as well as the angles between the bonds, known as bond angles, based on the BP and LP interactions. We can describe the molecular geometry, or the arrangement of bonded atoms in a molecule or polyatomic ion, using this information.
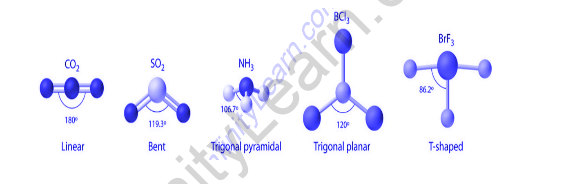
Postulates of VSEPR Theory:
The VSEPR theory’s postulates are listed below.
- In polyatomic molecules (molecules composed of three or more atoms), one of the constituent atoms is designated as the central atom, to which all other atoms in the molecule are linked.
- The total number of valence shell electron pairs determines the molecule’s shape.
- Electron pairs have a tendency to orient themselves in such a way that their electron-electron repulsion is minimised while their distance is maximised.
- The valence shell can be visualised as a sphere with electron pairs arranged on the surface to maximise the distance between them.
- An asymmetrically shaped molecule can be expected if the central atom of the molecule is surrounded by electron bond pairs.
- If the central atom is surrounded by both lone pairs and bond pairs of electrons, the molecule will be distorted.
- The VSEPR theory can be used to analyse the resonance structure of any molecule. The repulsion is strongest between two lone pairs and weakest between two bond pairs.
- If electron pairs near the central atom get too close, they will repel each other. As a result, the energy of the molecules increases.
Limitations of VSEPR Theory
The following are some significant limitations of the VSEPR theory:
- Isoelectronic species are not taken into account by this theory (i.e. elements having the same number of electrons). The morphologies of the species can differ despite having the same number of electrons.
- The VSEPR theory does not shed any light on transition metal compounds. This theory cannot accurately describe the structure of several such compounds.
- This is because the VSEPR theory does not account for the associated sizes of the substituent groups and the inactive lone pairs. Another limitation of VSEPR theory is that it predicts that halides of group 2 elements will have a linear structure when in fact they have a bent structure.
FAQs
Also read: Important Topic of Chemistry: Entropy
What is the VSEPR Theory's premise?
Because of the repulsion that exists between electron pairs in the valence shell, the atoms arrange themselves in such a way that this repulsion is minimised. This has an immediate impact on the geometry of the molecule formed by the atom.
What is the molecule's shape if the VSEP number is 5?
The molecule's structure would be trigonal bipyramidal.
What are the benefits of VSEPR theory?
This theory can be used to accurately predict the shapes of molecules in a wide range of compounds. Understanding the reactions of a molecule becomes easier once the geometry of the molecule is understood.






