Table of Contents
Introduction
Electron pair transfers are used to describe Lewis acids and bases. A Lewis base is indeed a donor of electron pairs, while a Lewis acid is an acceptor of electron pairs. An organic transition (the formation of products from reactants) is essentially the result of a process of bond breaking and bond formation. One such process is essentially a series of electron pair transfers.
The Lewis acid-base theory describes ionic mechanisms because they involve electron pair transfers. The Lewis concept implies the presence of high electron density centres in Lewis bases and low electron density centres in Lewis acids. The electron pair donated by the base is used to form a new sigma bond to the acid’s electron-deficient centre in a reaction between a Lewis acid and a Lewis base.
The recognition of Lewis bases follows the same principles as the identification of Bronsted bases. They often include atoms with nonbonding electrons, also known as lone pairs. Lewis acids, but in the other hand, frequently have atoms with an incomplete octet, a full positive charge, or a partial positive charge.
Overview
- Lewis acids and bases have been described as electron-pair acceptors and electron-pair donors, respectively, in the Lewis theory of acid-base reactions.
- As a result, a Lewis base can donate a pair of electrons to a Lewis acid, resulting in a product with a coordinate covalent bond. One such product is also known as a Lewis adduct.
- Lewis acids and bases have been named after American chemist Gilbert Newton Lewis, who also made significant contributions to thermodynamics and photochemistry.
Lewis Acid
Lewis acids seem to be chemical species with empty orbitals that can accept electron pairs from Lewis bases. Traditionally, this term was used to describe chemical species with a trigonal planar structure and an empty p-orbital. BR3 is an example of such a Lewis acid (where R can be a halide or an organic substituent).
Water and a few other compounds are both Lewis acids and bases because they can accept and donate electron pairs depending on the reaction.
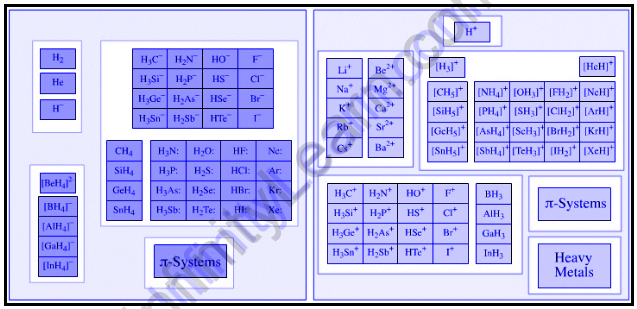
Few examples of Lewis acids that can accept electron pairs include:
- Together with onium ions such as H3O+, H+ ions (or protons) can be thought of as Lewis acids.
- High oxidation state cations of d block elements can act as electron-pair acceptors. Fe3+ is considered as an example of such a cation.
- Metal cations, such as Mg2+and Li+, can form coordination compounds with water as the ligand. These aqua complexes have the ability to accept electron pairs and act as Lewis acids.
- H3C+ and other trigonal planar species produce carbocations that accept electron pairs.
- Pentahalides of the following 15 elements can function as Lewis acids: antimony, arsenic, and phosphorus.
Aside from the above-mentioned chemical compounds, any electron-deficient system π, such as enones, can act as an acceptor of electron pairs.
Lewis Base
Lewis bases are atomic or molecular chemical species with a highly localized HOMO (The Highest Occupied Molecular Orbital). As previously discussed, these chemical species have the ability to donate an electron pair to a given Lewis acid in order to form an adduct.
Ammonia, alkyl amines, and other conventional amines are the most common Lewis bases. Lewis bases seem to be generally anionic in nature, and the pKa of the corresponding parent acid determines their base strength. Lewis bases are nucleophiles because they are electron-rich species with the ability to donate electron pairs. Lewis acids, too, can be classified as electrophiles (since they behave as electron-pair acceptors).
The following are examples of Lewis bases that can donate an electron pair.
- Pyridine and its derivatives are capable of acting as electron-pair donors. As a result, these compounds are Lewis bases.
- Lewis bases are compounds in which Oxygen, Sulphur, Selenium, and Tellurium (which belong to Periodic Group 16) have an oxidation state of -2. Water and ketones are two examples of such compounds.
- By donating their electrons, simple anions with an electron pair can also act as Lewis bases. H–and F– are two examples of such anions. Some complex anions, such as the sulphate-anion (SO42-), can also donate electron pairs.
- Electron-rich π-systems (such as benzene, ethylene, and ethene) have excellent electron pair donating capabilities.
Strong conjugate Lewis bases exist for weak Lewis acids. Aside from that, many chemical species with a single pair of electrons, such as CH3– and OH– , are classified as Lewis bases due to their ability to donate electron pairs.
Chemical Reactions Between Lewis Acids and Bases
(1) Reactions with the H+ ion:
The H+ ion functions as a Lewis acid, while H2O functions as a Lewis base. The reaction here between the water molecule and the proton results in the formation of a hydronium ion (H3O+).
In this case, the oxygen atom donates an electron pair to the proton, resulting in the formation of a coordinate covalent bond. The arising Lewis acid has a positive charge of +1. Some other reaction in which the H+ ion acts as a Lewis acid is the formation of ammonium ion (NH4+) from ammonia (NH3).
The proton receives an electron pair from the nitrogen atom in this reaction (belonging to the ammonia molecule). A Lewis adduct is formed when a coordinate covalent bond between the two is formed (the ammonium cation).
(2) Reaction Between Ag+ and Ammonia:
Two Lewis bases form an adduct with one Lewis acid in this reaction.
In this case, ammonia acts as a Lewis base, while silver ion acts as a Lewis acid. Every nitrogen atom donates an electron pair to Ag+, forming two distinct coordinate covalent bonds. The chemical formula of the adduct formed by the Lewis acid and base is Ag(NH3)2+.
(3) Reaction Between the Fluoride Ion and Boron Trifluoride:
A coordinate bond is formed between the fluoride anion (F–) and boron trifluoride in this reaction (BF3).
F– acts as an electron-pair donor in this case, while BF3 accepts the electron pair. A reaction here between Lewis acid and base produces an adduct with the chemical formula BF4–.
Applications of Lewis Acids and Bases
- Lewis acids act as a catalyst in the Friedel-Crafts reaction – AlCl3 accepts a lone pair of electrons from the chloride ion, resulting in the formation of AlCl4– in the Friedel-Crafts alkylation process.
- This results in the formation of the highly electrophilic carbonium ion, which functions as a strong Lewis acid.
- Lewis acids are widely used in organic chemistry to promote many cationic or pseudo-cationic chemical reactions.
- Lewis bases have quite a wide range of applications in modifying the selectivity and activity of metallic catalysts. Asymmetric catalysis is an important part of enantioselective synthesis in the production of pharmaceuticals. Chiral Lewis bases are frequently used to confer chirality on catalysts in order to enable asymmetric catalysis.
- A few Lewis bases can form numerous bonds with Lewis acids. Such compounds, also known as ‘multidentate Lewis bases’ or ‘chelating agents,’ have numerous industrial and agricultural applications.
FAQs:
Does Hydrochloric Acid qualify as a Lewis Acid?
Because it cannot accept an electron pair, hydrochloric acid cannot be classified as a Lewis acid. This compound, on the other hand, dissociates into its constituent ions, releasing H+ ions (which are considered as Lewis acids). Hydrochloric acid is often referred to as a classical acid rather than a Lewis acid due to its inability to accept electron pairs. Furthermore, when reacted with Lewis bases, HCl does not form any adducts.
Which metals act as Lewis Acids?
Because they contain one or more empty orbitals, metal ions such as Li+ and Mg2+ can accept pairs of electrons from a donating species. By accepting electron pairs from ligands, these ions tend to form coordination compounds. The majority of metal ions have a coordinated structure with some ligands. To donate an electron pair to a Lewis base, the metal ion must first dissociate from the ligand. These ions' Lewis adducts are typically complexes as well.
What are some examples of Lewis Bases?
Amines with the general formula R-NH3, the fluoride ion (F-), Ammonia (NH3), Water (H2O), Acetone and many other ketones.








