Table of Contents
The conformational investigation is the investigation of the different energy levels related to the various adaptations of a particle. Conformities are the different 3-layered plans that the particle can procure by uninhibitedly pivoting around σ-bonds. One should remember that adaptations are not isomers. Isomers are various particles. Adaptations are just unique underlying game plans of a similar particle.
Confirmation of Ethane
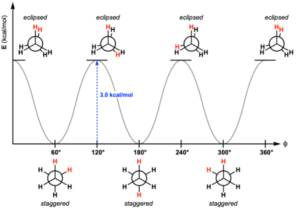
Ethane is a natural Chemical Compound. It is lacklustre and smells gas at a standard temperature. Ethane particle comprises of seven sigma bonds. There will be an adjustment of the state of the particle when there is a revolution of around six carbon-hydrogen bonds. However, numerous potential distinctions happen when there is a turn about the carbon-carbon bond.
Presently guess we pivot,
CH3 bunch clockwise at a point of 60 degrees, there would be a potential that hydrogen present at the front carbon is near the hydrogen present at the back carbon. That is Eclipsed Conformation.
Obscure Conformation is one of the greatest Conformation. One more clockwise revolution at a point of 60 degrees would prompt second overshadowed compliance. The strong line in the above figure addresses the 6 carbon-hydrogen bond that is reached out at a point of 120 degrees from 2 carbons.
 Utilizations Of Ethane: it is generally utilized in the development of Ethane. It is predominantly done through steam breaking. It goes about as an aging specialist for food. It is an essential fix in mustard gas.
Utilizations Of Ethane: it is generally utilized in the development of Ethane. It is predominantly done through steam breaking. It goes about as an aging specialist for food. It is an essential fix in mustard gas.
Conformation of Butane
Butane is a natural compound that comprises an alkane with 4 carbon particles. It might allude to a combination of 3 isomers. At air pressure, Butane is a gas. They are condensed gas that is exceptionally combustible.
Butane is an alkane with the presence of C-C bonds. Ordinarily, when we pivot the particle of butane at the hub of the C-C bond, it shows different adaptation isomerism. For the most part, Butane has four compliance isomers which are completely fully eclipsed, gauche, eclipsed, and anti butane conformational isomers.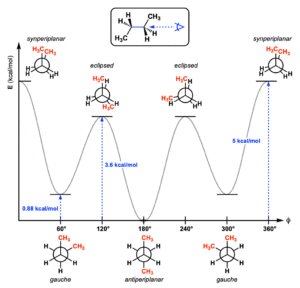
Utilizations of Butane: Pure of butane can be utilized as a refrigerant. It is utilized in Butane Torch. It is generally utilized in gas mixing. Butane Cartridges are utilized to controlled cordless hair irons.
FAQs
Which compliance of butane is generally steady?
In view of various steric connections, the most steady conformer is the counter conformer. The second-most stable conformer is the tactless conformer.
What is the counter compliance for butane?
So the dihedral point is 180 degrees. This compliance is known as counter adaptation. Also, the counter adaptation is least in expected energy, consequently, the counter conformity is the most steady conformity for butane.
Which adaptation is more steady in ethane and why?
Staggered adaptation The stunning adaptation is the most steady of all potential compliances of ethane since the points between C-H bonds on the front and back carbons are boosted which limits the energy.
What number of obscured adaptations does ethane have?
Two There are two significant conformities in ethane: the obscured and the stunning compliance. The Newman projection is a valuable theoretical instrument for picturing compliances.






