Table of Contents
The chemical compound with the formula XeF6 is xenon hexafluoride. This white crystalline chemical is one of three xenon binary fluorides, the others being XeF2 and XeF4. At normal temperatures, all are exergonic and stable. The strongest fluorinating agent in the series is XeF6. Xenon hexafluoride may be made by heating XeF2 at around 300 °C in the presence of fluorine at a pressure of 6 MPa (60 atmospheres). However, with NiF2 as a catalyst, this reaction can occur at 120 °C even with xenon-fluorine molar ratios as low as 1:5.
The intermixing of atomic orbitals of various shapes and approximately the same energy results in the same number of hybrid orbitals of the same shape, equal energy, and orientation, with little repulsion between these hybridized orbitals and is known as Hybridization.
Hybridization Types:
There are several forms of hybridization-based on orbital mixing.
- sp3 hybridization occurs when one s orbital and three p orbitals from the same shell of an atom combine to generate a new equivalent orbital.
- sp2 hybridization occurs when one s orbital and two p orbitals undergo energy mixing for analogous orbitals.
- When one s and one p orbital combine their energies to generate a new orbital, this is referred to as sp hybridization. Previously, molecules with sp hybridization had a linear structure with an angle of 180°.
- Along with the three fundamental hybridizations mentioned above, there are further hybridizations based on orbital mixings, such as sp3d hybridization, sp3d2 hybridization, and sp3d2 hybridization.
Hybridization Of XeF6
During hybridization of XeF6, the molecule is sp3d3 hybridized.
Using the usual formula, we can readily determine the hybridization of xenon hexafluoride;
Hybridization=1/2[V+M-C+A]
Where,
V = number of valence electrons,
M = monovalent
C = positive charge
A = negative charge
Let us input the values using the formula;
Hybridization = 1/2 [8+6-0+0]
= 1/2 [14]
= 7
The hybridization number is seven. We may now state that hybridization is sp3d3.
Alternatively, knowing the number of bond pairs and lone pairs allows us to calculate the hybridization. Xenon has 8 electrons in its valance shell during the production of XeF6, and it makes six bonds with the fluorine atoms. The molecules will also have one lone pair and six bond pairs. Now, if we take the steric number, it is 7. This is an example of sp3d3 hybridization.
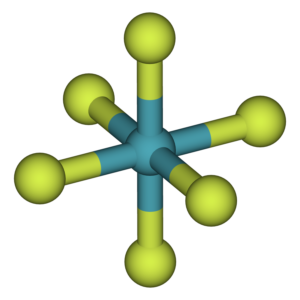
The chemical is monomeric in the gas phase. According to VSEPR theory, the structure lacks complete octahedral symmetry due to the presence of six fluoride ligands and one lone pair of electrons.
The geometry is a deformed octahedral (square bipyramidal) one rather than a pentagonal bipyramidal one. The fluorine atoms occupy the octahedron’s vertices, and lone pairs continue to move in space to minimize repulsion, altering the octahedral geometry.
FAQs
Q. Why is the hybrid orbital better than the parent atoms during hybridization?
Ans: The following are the reasons why a hybrid orbital is superior to its parents:
- Parent s; because, unlike the s orbital, it is directed.
- Parent p; Because it has less energy than the p orbital.
What exactly are hybrid orbitals?
Hybrid orbitals are formed by combining regular atomic orbitals and resulting in the production of new atomic orbitals.






