Table of Contents
Introduction
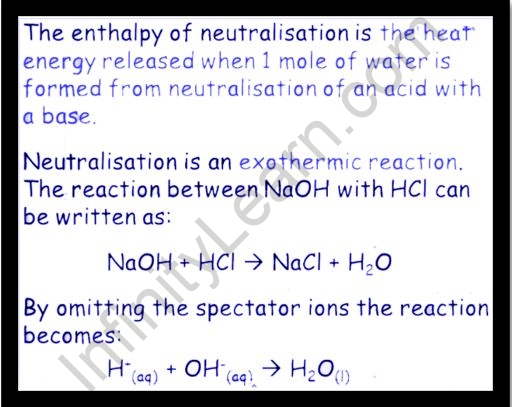
The change in enthalpy happens when one equivalent of an acid and a base undergo a neutralization reaction to generate water and salt is known as the enthalpy of neutralization (Hn). It is a specific case of reaction enthalpy. It is defined as the amount of energy released when one mole of water is formed.
A brief outline
The heat of neutralization for weak acids or bases is pH-dependent. Heat is required for full dissociation in the absence of any additional mineral acid or alkali. The overall amount of heat released during neutralization will be less. The standard enthalpy of neutralization occurs when a reaction is carried out under normal conditions at 298 K and 1 atm of pressure, resulting in the formation of one mole of water.
Important concepts
A neutralization reaction requires the interaction of H+ and OH- ions to make water when an acid and a base react to produce water and salt. The neutralization of a heavy acid and a strong base requires a pH of 7. When a strong acid and a weak base are neutralized, the resultant pH is less than 7, and when a strong base neutralizes a weak acid, the resultant pH is more than 7.
When a solution is neutralized, salts are created from equal weights of acid and base. The quantity of acid necessary is the same as one mole of protons (H+), and the amount of base required is the same as one mole of protons (H+) (OH-). Because salts are created through neutralization processes involving equal amounts of acid and base, N parts of the acid always will neutralize N parts of the base.
The indicator phenolphthalein is utilized in the neutralization reaction of most acid-base titrations. When combined in an acidic solution, this phenolphthalein is a colorless weak acid indication that remains colourless.
Significance of enthalpy of neutralization in IIT JEE exam
The topic under enthalpy of neutralization from the organic chemistry is worth around 8.33 per cent of the total 3 to 4 questions in the JEE test. The majority of the questions on this topic are answered using the NCERT textbook.
FAQs
When a solution is produced, the amount of heat energy emitted or absorbed is expressed in kJ / mol, and the enthalpy of solution or solution heat is expressed in kJ / mol.
It investigates the neutralization of a strong acid with a strong base and discovers that it always results in the release of heat into the environment. It's crucial to understand that during an exothermic reaction, bonds are formed and energy is released into the environment. As a result, the neutralization reaction is exothermic.
A chemical process in which heat is released is known as an exothermic reaction. A chemical reaction in which heat energy is captivated is identified as an endothermic reaction. Define the solution's enthalpy.
Determine whether the neutralizing process is an exothermic or an endothermic one.
What are the differences between the exothermic and endothermic reactions?






