Table of Contents
Phosphorus chloride could be a chemical compound with the formula PCl3. A colourless liquid once pure, it’s a vital industrial chemical, getting used for the manufacture of phosphates and different organophosphorus compounds. it’s virulent and reacts promptly with water to unleash acid.
PREPARATION
World production exceeds the tierce of 1,000,000 tonnes.[6] Phosphorus chloride is ready industrially by the reaction of halogen with white phosphorus, mistreatment of phosphorus chloride because of the solvent. during this continuous method, PCl3 is removed because it is made so as to avoid the formation of PCl5.
P4 + vi Cl2 → four PCl3
HYBRIDIZATION
In chemistry, orbital cross (or hybridization) is the conception of blending atomic orbitals to make new hybrid orbitals (with totally different energies, shapes, etc., then the element atomic orbitals) appropriate for the pairing of electrons to make chemical bonds in valence bond theory. for instance, in a very atom that forms four single bonds the valence-shell s orbital combines with 3 valence-shell p orbitals to make four equivalent sp3 mixtures in a very tetrahedral arrangement around the carbon to bond to four totally different atoms. Hybrid orbitals are helpful in the clarification of molecular pure mathematics and atomic bonding properties and are symmetrically disposed of in-house. typically hybrid orbitals are shaped by intermixture atomic orbitals of comparable energies.
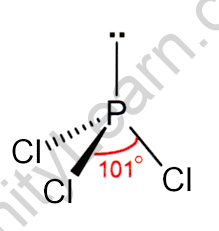
HYBRIDIZATION OF PHOSPHORUS TRICHLORIDE
If we glance at the molecule of PCl3 it’s created from phosphorus and halogen molecules but, the interbreeding primarily happens at intervals in the central atom which is phosphorus. currently, if we glance at the electronic structure within the state of phosphorus it’ll be 1s2, 2s2, 2p6, 3s2, 3p2. The valence shell in phosphorus is five. throughout the excited expressed one among the s electrons moves to AN empty d orbital leading to the modification in electronic configuration. In excited the electronic configuration can modification to 1s2, 2s2, 2p6, 3s2, 3px1, 3py1, 3pz1. Now, 3 odd electrons are accustomed type bonds with three halogen atoms. throughout the formation of the bond, four orbitals (3s2, 3px1, 3py1, 3pz1) can breed to create four equivalent sp3 hybrid orbitals. one among the hybrid orbitals can contain one lone try of electrons.
FAQs
What is the pure mathematics of PCl3?
Geometry: symmetrical pointed.
Does PCl3 and BCl3 have the same hybridization?
Dear student! each structure area unit totally different as a result, PCl3 may be a compound that has full and complete octet and then it's a stable structure with sp3 hybridizing and symmetrical pointed.






