Table of Contents
Structural Isomerism
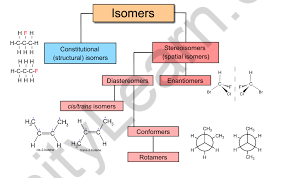
Organic chemistry is three-dimensional, which is one of its most intriguing characteristics. In space, a molecule can have a form that influences its characteristics. Molecules can differ in the way their atoms are ordered – the same atom combination can be formed in many ways. These substances are referred to as isomers. Isomers are molecules that have the same chemical formula but distinct atom configurations.
Structural isomers are isomers in which the atoms are arranged in a different order but have the same molecular formulae.
These are molecules with the same molecular formula but varied connectivities depending on the sequence in which they are assembled. The structure of Alkane (C4H10) is a basic example of a structural isomer with many isomers. The structural isomers rise as the number of carbon atoms in the alkane molecule increases.
In the instance of isomerism in organic chemistry, isomers do not always have comparable characteristics unless they also contain the same functional groups. Isomerism is most common in organic chemistry and has several applications in chemistry and medicine.
Structural Isomerism
- The structural isomers are isomers that differ in the atomic arrangement of the molecules without any relation to the spatial arrangement. Structural isomerism refers to the phenomena of structural isomers.
- According to the IUPAC, structural isomerism is also known as constitutional isomerism.
- It is a type of isomerism in which molecules with the same chemical formula but distinct ordering and bonding, as opposed to stereoisomerism.
Structural Isomerism Types
Structural isomerism is classified into three types: chain isomerism, position isomerism, and functional group isomerism.
- Chain Isomerism: Chain isomerism happens when the atomic arrangement of the carbon in a molecule’s carbon chain differs. Chain isomerism occurs when two or more compounds with the same kind of molecular formula but distinct primary chains display the feature. Skeletal isomerism is another name for this condition.
- Position Isomerism: Positional isomerism occurs when the locations of substituent atoms or a group of atoms change, or when unsaturation occurs in the chain. Location isomerism occurs when the position of functional groups with respect to the main chain atom changes.
- Functional Group Isomerism: Functional group isomerism arises when the odd form of functional groups with the same chemical formula coexist. Functional isomerism occurs when a substance has two distinct structures but the same chemical formula.
Structural isomerism is a form of isomerism in which the atoms and functional groups are linked in various ways (different positions).
FAQs
How Do We Find a Structural Isomer?
The bonding patterns of structural isomers can be used to identify them. The atoms in the compound are the same, but they are linked in such a way that they form different functional groups.
Give an example of a structural isomer and explain what it is.
Structural isomers are compounds that have the same molecular formula but have distinct atom arrangements or different bonds. Isobutane and butane, for example, contain the same amount of hydrogen and carbon atoms and so have the same chemical formula.