Table of Contents
Imperfection in solids refers to any abnormality in the pattern of crystal arrangement in a solid lattice. Defects develop when crystallization happens at a very fast or moderate pace.
Defects can be classified into two types:
- Point Defect
- Line Defect
Point Defect
A point defect develops when one atom is absent or is organized unevenly in a crystal lattice. In other terms, a Point Defect is defined as the deviations and abnormalities detected around an atom or a point.
Point Defects are categorized into two types:
- Defects in Stoichiometry: Stoichiometric Defects are those that cause the stoichiometry of solids to remain unchanged. In a solid, the cation-anion ratio remains constant after a stoichiometric defect.
Stoichiometric Defects are further subdivided into four categories:
- Vacancy Defect: The formation of vacancy defects occurs as a result of unoccupied lattice sites. This flaw usually appears when we heat a solid.
- Interstitial Defect: An interstitial defect occurs when some additional component particles become acquainted in the interstitial locations of a solid lattice.
- Schottky Defect: If an equal amount of cations and anions are absent from the lattice site in an ionic lattice, the lattice is said to have a Schottky defect. The electrical neutrality is preserved in the Schottky Defect, which often occurs in ionic materials.
- Frenkel Defect: Frenkel Defect arises when a smaller ion (usually a cation) is shifted from its typical location to a new interstitial site.
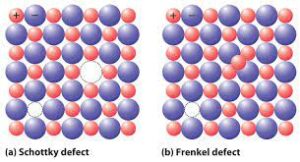
Non-ionic solids exhibit both vacancy and interstitial defects, whereas ionic solids exhibit these defects in the form of Schottky and Frenkel defects.
- Nonstoichiometric Defects: A non-stoichiometric defect is one in which the ratio of cation to anion in an ionic lattice varies. They are classified into two types:
- Metal Excess Defect: Metal Excess Defect manifests itself in two ways:
1. Because of anionic vacancies
Alkali halides such as NaCl and KCl exhibit Metal Excess defects. The anionic vacancies are lacking in this sort of defect and are filled by an electron to guarantee electrical neutrality. As a result of the existence of additional cations in interstitial locations
2. Due to the presence of extra cations at interstitial sites
Extra positive ions and electrons occupy interstitial sites and preserve electrical neutrality in this sort of defect.
- Metal Deficiency Defect: This defect occurs in metals that have fewer cations than anions. It is most commonly found in compounds with varying oxidation states. Metals obtained are non-stoichiometric due to an uneven amount of cations and anions.
Line Defects: A line defect is a flaw that extends across a tiny area along a row of the crystal lattice. Dislocations are linear flaws that cause atoms in the crystal lattice to be misaligned. Dislocations are of two types: edge dislocations and screw dislocations. “Mixed” dislocations, which include characteristics of both categories, are also prevalent.
FAQs
What is the point of imperfections?
Point Defect is another term for point imperfection. Imperfections or flaws in crystalline solids are classified into four types: line defect, point defect, volume defect, and surface defect. Crystal point flaws were originally recognized in ionic crystals, but not in metal crystals, which were considerably simpler.
What is the distinction between imperfections and defects in solids?
Any anomalies in the pattern of crystal arrangement in a solid lattice are considered imperfections in solids. Defects develop when crystallization (the formation of crystals) happens at a very fast or moderate pace.






