Table of Contents
Thermodynamics is the study of heat, work, and temperature in connection to energy, radiation, and the physical characteristics of matter. It discusses how thermal energy is transformed to or from other types of energy, as well as how this process affects matter. Thermal energy is energy derived from heat. The movement of microscopic particles within an item generates heat, and the quicker these particles move, the more heat is created.
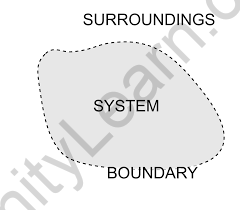
System
A thermodynamic system is a defined section of matter with a specified boundary on which our attention is focused. The system boundary might be real or fictitious, fixed or changeable.
There are three kinds of systems:
- Isolated System – An isolated system is incapable of exchanging both energy and mass with its surroundings. The universe is seen as a self-contained system.
- Closed System – Across the border of a closed system, energy is transferred but the mass is not transferred. Closed systems include refrigerators and the compression of gas in piston-cylinder assemblies.
- Open System – In an open system, both mass and energy can be transmitted between the system and its surroundings. An open system is exemplified by a steam turbine.
Surrounding
There is no one system in the world, but many. A system is a collection of elements or parts that are organized to achieve a common goal. The components of a system can be physical or abstract. The environment in which a system exists is also known as the system’s surroundings.
The physical environment is made up of the natural elements that surround a system. The climate, the land, the water, and the air are all part of the physical environment. The climate affects the way a system behaves, the type of land and water that is available, and the availability of natural resources.
The social environment is made up of the people and the institutions that interact with a system. The culture, the government, and the economy are all part of the social environment. The culture affects the way people think and behave, the government regulates the way a system works, and the economy provides the resources that a system needs.
The technological environment is made up of the tools and the technology that are used to interact with a system. The computers, the phones, and the internet are all part of the technological environment. The technology affects the way people communicate and the way a system works.
Thermodynamic Process
A thermodynamic process occurs when there is an energetic shift inside a system that is connected with changes in pressure, volume, and internal energy. There are four types of thermodynamic processes, each with its own set of characteristics, and they are as follows:
- Adiabatic Process – A method where no heat is transferred into or out of a system.
- Isochoric Process – A process in which there is no change in volume and the system does not perform any work.
- Isobaric Process – A process in which there is no change in pressure.
- Isothermal Process – A process in which there is no change in temperature.
A thermodynamic cycle is a series of operations that are carried out in such a way that the system’s beginning and end states are the same. A thermodynamic cycle is sometimes referred to as a cyclic operation or a cyclic process.
FAQs
Explain the concept of thermodynamics.
The phenomena of thermodynamics is the study of interactions involving heat, mechanical work, and other elements of energy transfer that occur in devices such as refrigerators, heat pumps, and internal combustion engines, among others.
What is the significance of thermodynamic laws?
The thermodynamic laws specify physical variables such as temperature, energy, and entropy that characterize thermodynamic systems in thermal equilibrium.






